FIGURE 4.
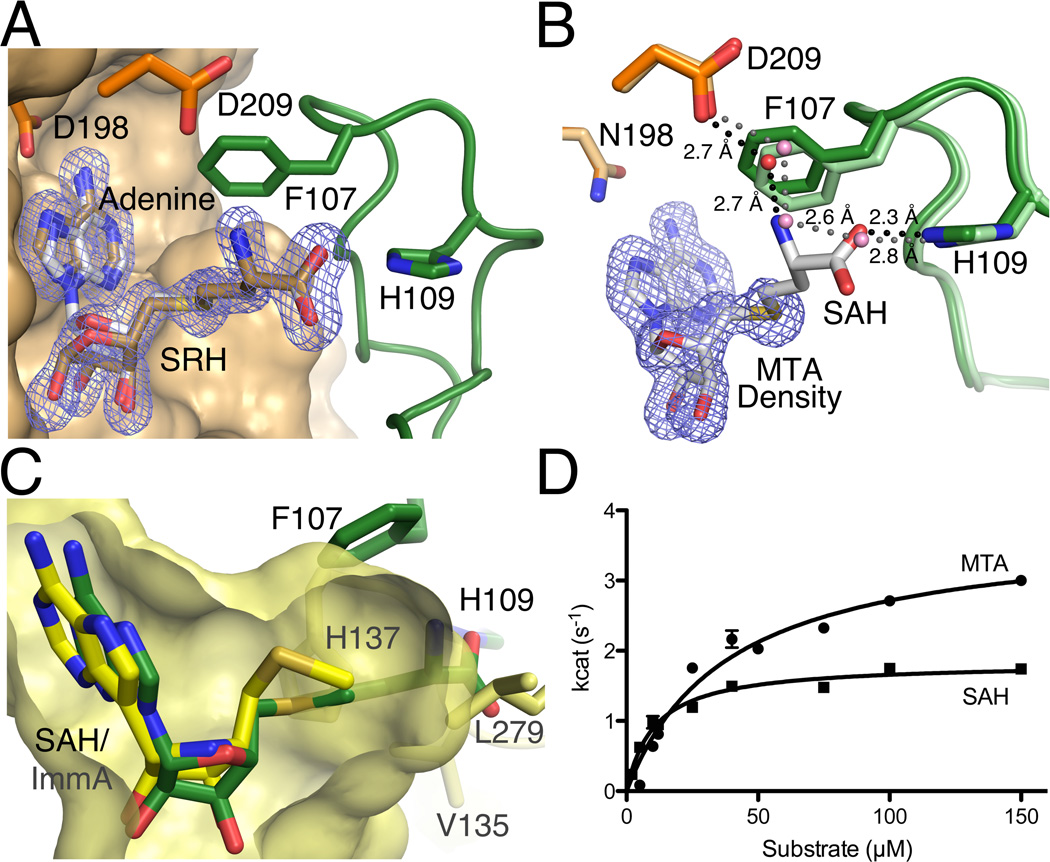
Comparison of SAH and MTA as substrates. A. Shown is the superposition of the HpMTAN-D198N/SAH and wt-HpMTAN/SRH/adenine complex structures. The Fo-Fc difference density from the wt-HpMTAN/SRH/adenine diffraction data is shown contoured at 3σ. Both SRH and adenine were omitted during map calculation. Coloring of the protein and SAH is the same as previous figures. The bronze carbon atoms represent the SRH and adenine products. B. Shown is the superposition of the HpMTAN-D198N/SAH and MTA complex structures. The protein atoms from the HpMTAN-D198N/SAH structure is colored as in previous figures and the carbon atoms of the enzyme of the MTA complex structure are slightly lighter in color. Since SAH and MTA atom positions superimpose perfectly, the atoms of MTA are not shown but the location of MTA is indicated by an Fo-Fc omit map contoured at 3σ where MTA was omitted from the map calculation. The hydrogen bonded water network in the MTA bound structure is indicated by the dashed gray lines and that of the SAH complex structure is indicated by the dashed black lines. C. HpMTAN and human MTAP 5’-alkylthio binding subsites differ in structure. A superposition of the HpMTAN-D198N/SAH complex active site with the human MTAP active site showing bound methylthio-Immucillin-A (1K27). Components of the HpMTAN-D198N/SAH complex are colored as shown previously. The corresponding 5’-alkylthio binding subsite of human MTAP is shown in yellow. The yellow carbon atoms and surface indicate the MTAP/methylthio-Immucillin-A (ImmA) complex and the active site cavity, respectively. H137, V135 and L279 form the boundary of the 5’-alkylthio binding subsite in MTAP. H109, F107 and SAH from the HpMTAN-D198N/SAH complex are also shown. The homocysteine moiety of SAH protrudes more than 4Å beyond the confines of the human MTAP active site. D. Comparison of steady-state kinetics using either MTA or SAH shows a slightly higher affinity for SAH but lower enzyme turnover when compared to the use of MTA. Error bars are the result of reactions performed in triplicate.