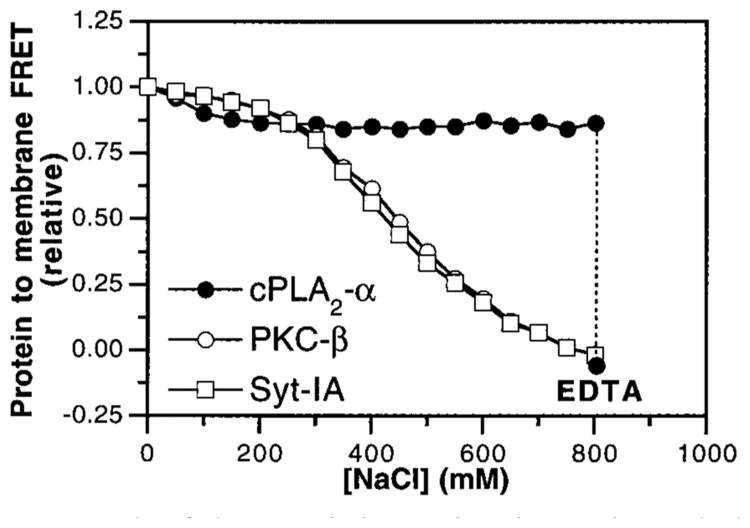
Figure 9.
Role of electrostatic interactions in membrane docking by C2 domains. 1 mM free Ca2+ was added to solutions containing the cPLA2-α (filled circles), PKC-β (open circles), and Syt-IA (open squares) C2 domains and vesicles of PS–PC–dPE (47.5%:47.5%: 5%) in assay buffer lacking added KCl. Membrane docking was measured by monitoring protein-to-membrane FRET; all subsequent FRET signals were corrected for small effects of NaCl on dPE emission. The NaCl concentration was raised incrementally, and the resulting FRET signal was normalized to the initial value. Following the NaCl additions, excess EDTA was added (dashed line) to demonstrate the reversibility of cPLA2-α C2 domain binding to membranes. Experimental conditions: 25 °C; 20 mM HEPES, pH 7.4, 5 mM DTT, less than 10 mM KCl, 1 mM EDTA, and 250 μM phospholipid.