Highlights
► Ovarian cancer presents as thoracic vertebral lesion in the absence of gross abdominal disease ► Bilateral salpingo-oophorectomy with surgical resection of vertebral lesion leaves patient with no clinical evidence of disease ► Ovarian cancer can present as vertebral metastases in the absence of pelvic or abdominal metastatic disease
Keywords: Ovarian cancer, Vertebral, Metastases
Introduction
Ovarian cancer is the second most common gynecologic malignancy and the most common cause of death among women with gynecologic cancer. Epithelial ovarian cancer is historically known as the “silent killer” due to symptoms that would not occur until very late in the course of the disease. Advanced ovarian cancer typically presents with abdominal distention, nausea, anorexia, or early satiety due to the presence of ascites and omental or bowel metastases. Dyspnea can occasionally be present due to a pleural effusion. The main route of dissemination in ovarian cancer is through a transcoelemic or lymphatic spread. Hematogenous spread is uncommon for this disease.
Ovarian cancer with bone metastasis is a relatively rare occurrence. Rose et al. in an autopsy study showed that metastasis to bones from these tumors is rare (0.1–0.12%). This and other case reports have shown that when bone metastases occur they often appear as recurrent disease or in conjunction with other abdominal metastases (Rose et al., 1989; Cormio et al., 2003). Other case reports have described ovarian cancer presenting initially with bone metastases prior to primary staging. These cases, however, had clinically significant abdominal or pelvic disease existing simultaneously that did not cause symptoms (Dvoretsky et al., 1989; Chen et al., 2009). Bony metastases existing on initial presentation in the absence of significant abdominal disease has not been observed thus far to our knowledge.
Case report
A 47 year-old, G4P2022, with a prior total abdominal hysterectomy for leiomyomatous uterus, presented with back pain over her right rib cage. There were no associated abdominal complaints. Magnetic resonance imaging of the thoracic spine revealed a mass at T10 with abnormal enhancement that extended from the vertebral body and the posterior elements, into the 10th rib — concerning for neoplastic involvement (Picture 1). Para-spinal core biopsy of the lesion was positive for adenocarcinoma with immunohistochemical stains positive for CK7 and WT-1. Staining for CK20, TTF-1 and CEA were all negative. This was compatible with a high-grade serous carcinoma of the gynecologic tract. The patient was promptly referred to gynecologic oncology for evaluation. She underwent a bilateral salpingo-oophorectomy, appendectomy, and omental sampling. A normal upper abdominal survey was noted intra-operatively. The ovaries themselves were grossly normal. The histopathologic report was positive only for a 3 mm micro-foci of serous carcinoma on the left ovarian surface, staining positive for WT-1 and p53 — similar to her known focus of carcinoma from her para-spinal core biopsy. The omentum, appendix, and pelvic washings were all negative for malignant cells. The patient was diagnosed with Stage IVB serous ovarian carcinoma with no identifiable involvement other than the thoracic vertebral lesion and small foci of malignant tissue on the left ovary. The patient's CA-125 level was 56.6 U/ml.
Picture 1.
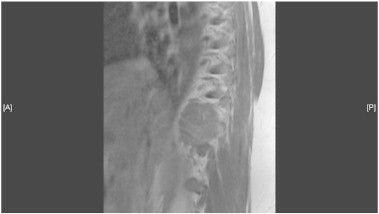
Sagittal MRI image of T10 metastatic lesion.
The patient was referred to the spinal oncology service for resection of the vertebral mass. She underwent an intralesional en-bloc resection of the tumor, using a posterior costotransversectomy approach. To obtain adequate margins three ribs were removed as well as portions of her parietal pleura. A chest wall reconstruction was performed using a Gore-tex graft. A posterior spinal fusion and decompression from T8–T12 was performed with instrumentation. Surgical pathology revealed high-grade serous adenocarcinoma at the T10 level (Picture 2). Rib and pleural tissue were all negative for malignant cells. The histology was consistent with pathology from the gynecologic tract. The patient's post-operative recovery was complicated by a right hemothorax, requiring 2 chest tube placements, prolonging her hospital stay; this complication resolved leading to a full surgical recovery. Post-operatively the patient underwent an adjuvant course of carboplatin/paclitaxel along with stereotactic radiation (8 Gy × 3) to the spine. At the time of the drafting of this manuscript, the patient has completed her adjuvant chemotherapy regiment. She is disease free as demonstrated by her post-operative MRI (Picture 3). Her most recent CA-125 level is 8.8 U/ml. She has no clinical evidence of disease.
Picture 2.
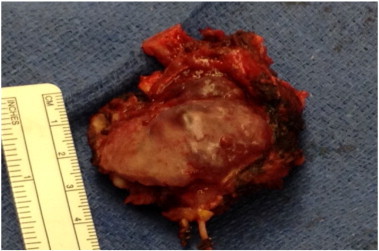
Resection of T10 mass along with section of rib. Histopathology revealed high grade serous carcinoma of ovarian origin.
Picture 3.
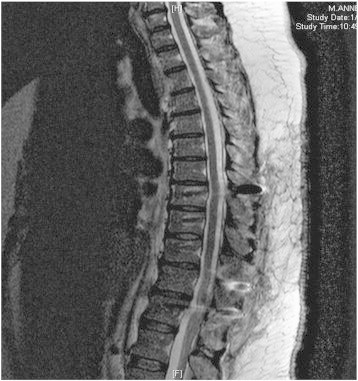
Post-operative MRI.
Discussion
Ovarian epithelial malignancies usually spread by direct extension, trans-peritoneal seeding or lymphatic spread. Distant metastases are present in only 8% of the patients at the time of their diagnosis. Twenty-two percent of patients present with distant metastases during the course of the disease (Cormio et al., 2003). Bone metastases from epithelial ovarian malignancies have rarely been reported in the literature. Rose et al. in their autopsy series reviewed the metastatic pattern in 428 ovarian cancers and correlated different histologic sub-types with patterns of spread. The incidence of bony metastasis was 0.06–0.19% for epithelial histology, highlighting the rarity of bone involvement in this form of malignancy (Rose et al., 1989). The mode of spread appears to be hematogenous although no definitive route of spread has been defined.
In an autopsy series, Dauplat et al. analyzed 336 patients of distant metastasis from ovarian cancers. Four patients had bony metastases, 2 of which were present in the thoracic vertebra. None of the patients in this series had bone metastasis as a first site of presentation. According to the authors, bony metastases are the rarest known first site for metastatic disease with a median time for development ranging from 13 to 49 months (Dvoretsky et al., 1989). The vertebral bodies are the most common sites for metastasis, followed by the ribs, clavicle, skull and femur.
When bony metastases do occur they are rarely isolated findings. In situations where there are isolated findings they are generally present as recurrent disease in previously staged patients (Gottwald et al., 2012). Tiwari et al. reported a case of ovarian cancer metastasizing to the lumbar vertebrae (Tiwari et al., 2007). The lumbar metastasis in this case happened in conjunction with hepatic lesions, as a recurrent presentation in a patient that had previously been diagnosed with stage IIIC ovarian carcinoma. Chen et al. also reported a case of back pain from vertebral metastasis presenting as the initial complaint in a patient with ovarian adenosarcoma (Chen et al., 2009). However, in this patient significant abdominal disease was noted on surgical staging. Kingston et al. also reported on a patient who presented with right shoulder pain from an ovarian metastasis to the humeral head but further work up revealed significant abdominal and thoracic pathology as well (Kingston et al., 2001). Bone involvement in ovarian cancer is associated with a poor prognosis. Kumar et al. reported a median survival of 7.5 months (range 6–39 months) in patients with bony involvement (Kumar et al., 1992). The use of direct surgical resection plus post-operative radiotherapy has been shown to be superior to radiotherapy alone in patients with spinal metastases (Patchell et al., 2005).
In the present case, we report a patient with an initial presentation of back pain from vertebral metastasis that lacked any gross disease in her pelvis or abdomen during her staging procedure. This is an extremely rare method for ovarian cancer to initially present and, as far as we know, has never been reported.
Conflict of interest statement
No conflict of interest.
References
- Rose P.G., Piver S.M., Tsukada Y., Lau T.S. Metastatic patterns in histologic variants of ovarian cancer: an autopsy study. Cancer. 1989;64:1508–1513. doi: 10.1002/1097-0142(19891001)64:7<1508::aid-cncr2820640725>3.0.co;2-v. (Back to cited text no. 5) [DOI] [PubMed] [Google Scholar]
- Cormio G., Rossi C., Cazzolla A., Resta L., Loverro G., Greco P., Selvaggi L. Distant metastasis in ovarian carcinoma. Int. J. Gynecol. Cancer. 2003;13:125–129. doi: 10.1046/j.1525-1438.2003.13054.x. [DOI] [PubMed] [Google Scholar]
- Dvoretsky P.M., Richards K.A., Bonfiglio T.A. The pathology and biologic behavior of ovarian cancer. An autopsy review. Pathol. Annu. 1989;24:1–24. (Back to cited text no. 4) [PubMed] [Google Scholar]
- Chen Y.L., Hsiao S.M., Lin M.C., Lin H.H. Bone metastasis as the initial presentation in one case of ovarian cancer with two components of endometrioid adenocarcinoma and adenosarcoma. Taiwan J. Obstet. Gynecol. 2009;48(3):298–301. doi: 10.1016/S1028-4559(09)60309-0. [DOI] [PubMed] [Google Scholar]
- Gottwald L., Dukowicz A., Piekarski J., Misiewicz B., Spych M., Misiewicz P., Kazmierczak-Lukaszewicz S., Moszynska-Zielinska M., Cialkowska-Rysz A. J. Obstet. Gynaecol. 2012;32(1):81. doi: 10.3109/01443615.2011.619672. [DOI] [PubMed] [Google Scholar]
- Tiwari A., Kumar N., Bajpai R., Lal P. Bone metastasis from ovarian cancer. J. Cancer Res. Ther. 2007;3:34–36. doi: 10.4103/0973-1482.31969. [DOI] [PubMed] [Google Scholar]
- Kingston R., Sparkes J., Leen E., Stafford-Johnson D., Keogh P. Bone metastasis as the presenting complaint in ovarian carcinoma. Acta Obstet. Gynecol. Scand. 2001;80:669–670. [PubMed] [Google Scholar]
- Kumar L., Bhargava V.L., Rao R.C., Rath G.K., Kataria S.P. Bone metastasis in ovarian cancer. Asia Oceania J. Obstet. Gynaecol. 1992;18(4):309–313. doi: 10.1111/j.1447-0756.1992.tb00324.x. (Dec) [DOI] [PubMed] [Google Scholar]
- Patchell R.A., Tibbs P.A., Regine W.F., Payne R., Saris S., Kryscio R.J., Mohiuddin M., Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366(9486):643. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]


