Highlights
-
•
This is the first case report of inflammatory myofibroblastic tumor in the literature to present with extrauterine disease.
-
•
A prompt work-up of symptoms may have precluded a tumor debulking procedure.
Keywords: Inflammatory myofibroblastic tumor, Tumor debulking, Uterine cancer
Introduction
Inflammatory myofibroblastic tumor (IMT) is a neoplasm of low malignant potential (Shintaku and Fukushima, 2006). It is composed of mesenchymal cells showing differentiation toward myofibroblast or fibroblast and is usually associated with an inflammatory cell infiltration within the lesion (Coffin et al., 1995; Pettinato et al., 1990). IMT was first described in the lung and most commonly involves that organ, however, it may occur in a wide range of organs including the retroperitoneum, mesentery, extremities, head, neck, omentum, and genitourinary system (Coffin et al., 2007; Olgan et al., 2011). It has been previously referred to as inflammatory pseudotumor, plasma cell granuloma, or pseudosarcomatous myofibroblastic proliferation (Shintaku and Fukushima, 2006; Coffin et al., 2007; Rabban et al., 2005). A triad of findings define IMT: 1) myofibroblastic proliferation lacking nuclear atypia; 2) lymphoplasmacytic infiltrate; 3) variable myxoid stromal background (Rabban et al., 2005). IMT was considered a reactive or inflammatory lesion, but cytogenetic studies have shown genetic rearrangements of the anaplastic lymphoma kinase (ALK) gene located in the chromosomal region 2p23 of this lesion confirmed its true neoplastic nature (Olgan et al., 2011; Rabban et al., 2005; Butrynski et al., 2010). This genetic rearrangement is seen in approximately 50% of IMT and is considered a useful diagnostic marker (Shintaku and Fukushima, 2006). The prognosis of IMT is generally favorable, but can vary. Rare cases are known to involve malignant transformation. Unfortunately, there are no definitive morphological criteria to predict an aggressive clinical behavior in these types of tumors. The presence of large cells with “ganglion-like” features suggests a more aggressive clinical behavior (Shintaku and Fukushima, 2006).
Uterine involvement by IMT is rare. To our knowledge only 12 cases has been reported in the literature (Shintaku and Fukushima, 2006; Olgan et al., 2011). The histologic appearance of uterine IMT is similar to that of extra-uterine IMT. Clinically, the majority of IMT's are benign, but they require adequately surgical removal because there is a propensity for local recurrence. The cases of IMT reported in the literature involving gynecologic organs are confined to the uterus. To our knowledge, this is the first report of IMT that spread beyond the uterus to involve other gynecologic and intraperitoneal organs.
Case report
A 30-year-old Para 0 woman started to experience irregular menstrual periods in 2010. Physical examination including pelvic exam was normal. A pelvic ultrasound from 2010 showed a retroverted uterus that measured 6.0 × 4.5 × 1.6 cm. The endometrium measured 11 mm with a cystic area measuring 11.5 mm. The right ovary measured 2.4 × 1.9 × 1.7 cm. The left ovary measured 2.5 × 1.8 × 1.6 cm. She was started on depo-provera with resolution of symptoms. In June of 2012, she developed left lower quadrant pain, post-coital bleeding and irregular menstrual periods that were heavy associated with passage of blood clots and soaking of pads. A pelvic ultrasound showed a uterus that measured 8.4 × 4.5 × 5.3 cm. Endometrium was 15 mm with fluid within the lower portion of the uterus. The right ovary measured 2.9 × 2.4 × 1.9 cm. The left ovary measured 2.5 × 1.8 × 1.8 cm with an echogenic area surrounding the left ovary measuring 7.2 × 6.2 × 3.8 cm. A CT scan of the abdomen and pelvis with PO and IV contrast showed a complex, left adnexal mass that measured 6.3 × 5.0 × 6.1 cm. Multiple, small, low-density masses were present within the right pelvis and midline location measuring less than or equal to 1.8 cm in size. Uterus was not enlarged. No lymphadenopathy was appreciated. PAP smear from April 2012 was negative. She underwent an examination under anesthesia with cystoscopy, proctoscopy, cervical polypectomy, hysteroscopy, dilatation and curettage, laparoscopy, and excision of pelvic masses in July of 2012 at an outside facility. Intraoperative findings revealed polypoid masses within the uterus, friable tissue on the cervix, and multiple polypoid masses in the pelvis adherent to the bowel and mesentery. Masses were noted bilaterally in the pelvis, and within the cul-de-sac (see Image 1). There was a 2.3 cm pedunculated mass extending off the inferior portion of the cervix with multiple smaller masses extending from the anterior lip of the cervix. These masses were friable and bled easily. The rectum was free of disease up to 17 cm. Cystoscopy was normal. Pathology revealed an IMT. Immunohistochemical stains were performed to confirm the diagnosis. The tissue specimen was smooth muscle actin: negative, muscle specific: negative, Desmin: positive, ALK-1: negative, HMB-45: negative, CD34: negative, CD117: negative, Cathespsin-k: positive, ER: positive, PR: positive (see Fig. 1).
Fig. 1.
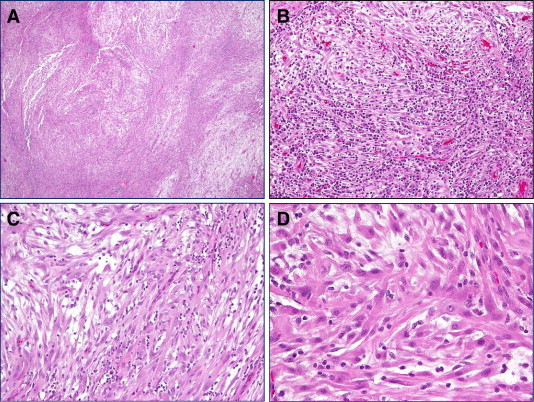
The histology of the tumor from this case. (A) A low magnification show fascicles of spindle cell neoplasm with hypercellular area alternating with hypocellular and edeomous tumor region. (B and C) A medium magnification reveals spindle cells intermingled with prominent inflammatory cell infiltrates. (D) A high magnification demonstrates fibroblast-like tumor cells with abundant eosinophilic cytoplasm. Inflammatory cells are clearly evident.
A 30-year-old Para 0 woman started to experience irregular menstrual periods in 2010. Physical examination including pelvic exam was normal. A pelvic ultrasound from 2010 showed a retroverted uterus that measured 6.0 × 4.5 × 1.6 cm. The endometrium measured 11 mm with a cystic area measuring 11.5 mm. The right ovary measured 2.4 × 1.9 × 1.7 cm. The left ovary measured 2.5 × 1.8 × 1.6 cm. She was started on depo-provera with resolution of symptoms. In June of 2012, she developed left lower quadrant pain, post-coital bleeding and irregular menstrual periods that were heavy associated with passage of blood clots and soaking of pads. A pelvic ultrasound showed a uterus that measured 8.4 × 4.5 × 5.3 cm. Endometrium was 15 mm with fluid within the lower portion of the uterus. The right ovary measured 2.9 × 2.4 × 1.9 cm. The left ovary measured 2.5 × 1.8 × 1.8 cm with an echogenic area surrounding the left ovary measuring 7.2 × 6.2 × 3.8 cm. A CT scan of the abdomen and pelvis with PO and IV contrast showed a complex, left adnexal mass that measured 6.3 × 5.0 × 6.1 cm. Multiple, small, low-density masses were present within the right pelvis and midline location measuring less than or equal to 1.8 cm in size. Uterus was not enlarged. No lymphadenopathy was appreciated. PAP smear from April 2012 was negative. She underwent an examination under anesthesia with cystoscopy, proctoscopy, cervical polypectomy, hysteroscopy, dilatation and curettage, laparoscopy, and excision of pelvic masses in July of 2012 at an outside facility. Intraoperative findings revealed polypoid masses within the uterus, friable tissue on the cervix, and multiple polypoid masses in the pelvis adherent to the bowel and mesentery. Masses were noted bilaterally in the pelvis, and within the cul-de-sac (see Image 1). There was a 2.3 cm pedunculated mass extending off the inferior portion of the cervix with multiple smaller masses extending from the anterior lip of the cervix. These masses were friable and bled easily. The rectum was free of disease up to 17 cm. Cystoscopy was normal. Pathology revealed an IMT. Immunohistochemical stains were performed to confirm the diagnosis. The tissue specimen was smooth muscle actin: negative, muscle specific: negative, Desmin: positive, ALK-1: negative, HMB-45: negative, CD34: negative, CD117: negative, Cathespsin-k: positive, ER: positive, PR: positive (see Fig. 1).
The patient underwent an examination under anesthesia with exploratory laparotomy, modified radical hysterectomy with bilateral salpingo-oopherectomy, resection of left pelvic sidewall mass, resection of pelvic peritoneum, resection of rectosigmoid implants, resection of bladder peritoneum, and cystoscopy. All visible disease was removed. The patient's post-operative course was uneventful. She was discharged home on postoperative day #2. She recovered well from the surgery. The final pathology confirmed the diagnosis of IMT. Pathology revealed that the tumor involved the uterus, cervix, bilateral fallopian tubes and ovaries, left pelvic sidewall mass, bladder and rectosigmoid peritoneum, and parametrium. The histology of the tumor is shown in Fig. 1. At the present time, the patient is six months out from surgery and has no evidence of disease.
Discussion
IMT of the uterus is rare. Although the lung is the most common site of occurrence, it does occur in diverse extrapulmonary locations (Coffin et al., 1995). Regardless of the site of origin, IMT has a predilection for younger patients (Coffin et al., 1995; Olgan et al., 2011). The age range of women affected by IMT was 6–63 years of age, with the majority of women being diagnosed in the first four decades of life (Shintaku and Fukushima, 2006; Olgan et al., 2011; Rabban et al., 2005; Azuno et al., 2003; Gilks et al., 1987; Gupta et al., 2011). Symptoms at presentation were varied including intermittent abdominal pain, dyspareunia, abdominal distention, new onset menorrhagia, fever, and weight loss.
Surgical approach to this disease varies based on the extent of disease, age of the patient, and suggested pathologic findings. The extent of surgery has ranged from minor procedures like hysteroscopy and dilation and curettage to exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oopherectomy (Shintaku and Fukushima, 2006; Olgan et al., 2011). Surgical resection is the treatment of choice. In 90% or greater cases of IMT, excision is curative, including the amelioration of associated constitutional symptoms within days or few weeks of the resection (Rabban et al., 2005). Most patients have no recurrence or metastasis within 5 years (Rabban et al., 2005). The 10% of patients that recur have a large burden of disease at the onset, and will require surgical management for recurrent disease, as chemotherapy and irradiation therapy does not seem to be of use (Coffin et al., 1995). The role of hormonal therapy in this setting is unknown due to a paucity of literature.
The greatest clinical concern is the misdiagnosis of an IMT as an aggressive mesenchymal neoplasm, like leiomyosarcoma or myxoid leiomyosarcoma, since uterine IMTs appear to be indolent (Olgan et al., 2011). IMT can be differentiated from leiomyosarcoma by a low mitotic rate, the absence of atypical mitotic figures or nuclear atypia, and by the absence of coagulative tumor cell necrosis. It is more difficult to differentiate IMT from myxoid leiomyosarcoma. Their morphologic features are similar with the exception that myxoid leiomyosarcoma has an infiltrative microscopic appearance; irregular tongues of tumor invading into adjacent tissue and blood vessels (Rabban et al., 2005).
Conclusion
Although IMT has a low malignant potential, it is important to identify this process early as to minimize the amount of surgical intervention needed. As in this case, had the patient been further evaluated when she first presented with symptoms, the need for a total abdominal hysterectomy, bilateral salpingo-oopherectomy, and tumor debulking might have been avoided.
The following is the supplementary data related to this article.
Intraperitoneal disease.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gynor.2013.07.007.
Conflict of interest statement
We have no conflict of interests to declare.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Azuno Y., Yaga K., Suehiro Y. Inflammatory myoblastic tumor of the uterus and interleukin-6. Am. J. Obstet. Gynecol. 2003;189:890–891. doi: 10.1067/s0002-9378(03)00208-4. [DOI] [PubMed] [Google Scholar]
- Butrynski J.E., D'Adamo D.R., Hornick J.L. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. NEJM. 2010;363:1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin C.M., Watterson J., Priest J.R. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am. J. Surg. Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- Coffin C.M., Hornick J.L., Fletcher C.D. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am. J. Surg. Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- Gilks C.B., Taylor G.P., Clement P.B. Inflammatory pseudotumor of the uterus. Int. J. Gynecol. Pathol. 1987;6:275–286. doi: 10.1097/00004347-198709000-00008. [DOI] [PubMed] [Google Scholar]
- Gupta N., Mittal S., Renu Misra. Inflammatory pseudotumor of uterus: an unusual pelvic mass. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;156:118–119. doi: 10.1016/j.ejogrb.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Olgan S., Saatli B., Okyay R.E. Hysteroscopic excision of inflammatory myofibroblastic tumor of the uterus: as case report and brief review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:234–240. doi: 10.1016/j.ejogrb.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinato G., Manivel J.C., De Rosa N. Inflammatory myofibroblastic tumor (plasma cell granuloma). Clinicopathologic study of 20 cases with immunohistochemical and ultrastructural observations. Am. J. Clin. Pathol. 1990;94:538–546. doi: 10.1093/ajcp/94.5.538. [DOI] [PubMed] [Google Scholar]
- Rabban J.T., Zaloudek C.J., Shekitka K.M. Inflammatory myofibroblastic tumor of the uterus: a clinicopathologic study of 6 cases emphasizing distinction from aggressive mesenchymal tumors. Am. J. Surg. Pathol. 2005;29:1348–1355. doi: 10.1097/01.pas.0000172189.02424.91. [DOI] [PubMed] [Google Scholar]
- Shintaku M., Fukushima A. Inflammatory myofibroblastic tumor of the uterus with prominent myxoid change. Pathol. Int. 2006;56:625–628. doi: 10.1111/j.1440-1827.2006.02018.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraperitoneal disease.


