Abstract
Elevated serum levels of several cytokines have been reported in ovarian cancer. We have previously found a diagnostic and prognostic value of hepatocyte growth factor (HGF).
The aims of this study were to evaluate the diagnostic and prognostic value of multiple serum cytokines in women with ovarian tumors, and to examine possible associations between serum levels of cytokines and the previously analyzed HGF. Preoperative levels of multiple cytokines were quantified by serum-based immunoassays in 113 women with a pelvic mass: 57 carcinomas, 23 borderline tumors, and 33 benign ovarian tumors. The results were related to clinicopathological parameters. Univariate and multivariate analyses of five-year overall survival were performed.
The women with ovarian carcinoma had significantly higher preoperative serum levels of cancer antigen 125 (CA 125), interleukin 8 (IL-8), and plasminogen activator inhibitor-1 (PAI-1) than women with benign ovarian tumors. Serum IL-8 and PAI-1 levels were positively correlated to serum levels of HGF. In a multivariate analysis of five-year overall survival, IL-8 had a prognostic impact.
Serum levels of IL-8 and PAI-1 were elevated in women with ovarian carcinoma compared to women with benign ovarian tumors, and positively correlated to serum HGF levels in women with ovarian tumors. IL-8 also seemed to have a prognostic impact.
Keywords: Ovarian cancer, Plasma markers, Interleukin-8, Plasminogen activator inhibitor-1, Hepatocyte growth factor
1. Introduction
Ovarian cancer is the most lethal gynecological malignancy in the Western world. Although the survival has improved during the last decade, only 40–45% of the patients are still alive five years after diagnosis. Whereas the five-year survival is favourable for women with early stage disease (about 90%), survival with advanced disease is only around 20% [1]. Unfortunately, most cases are not diagnosed until the disease is in an advanced stage. Early diagnosis would probably contribute to a drastic reduction in mortality. Different screening strategies based on tumor markers have been evaluated, but an effective screening method has so far not been established. CA 125 is the most used marker in the management of ovarian cancer [2,3], and is detected in serum of more than 80%. It is, however, highly unspecific, and can be elevated due to other conditions, both benign and malignant [4].
We have recently shown the diagnostic and prognostic value of HGF in ovarian epithelial cancer [5]. HGF is a multifunctional growth factor that enhances cell proliferation, motility, and angiogenesis [6]. Elevated HGF levels have been described in several human cancers [7–10]. There are, however, few reports on possible associations between serum levels of HGF and other cytokines in women with ovarian cancer.
Active signal substances in inflammatory processes, such as cytokines, growth factors, and prostaglandins, are involved in the pathogenesis of ovarian cancer [3,11], and elevated serum levels of several cytokines have been reported in ovarian cancer [12–15]. Because no single marker has shown adequate validity in screening for ovarian cancer alone, research has been directed toward multi-marker panels [16]. A diagnostic value of the combination of CA 125, IL-6, IL-8, vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and monocyte chemotactic protein-1 (MCP-1) was, for example, found with this approach [12,17].
The aims of the present study were to analyze the validity of multiple cytokines in predicting epithelial ovarian cancer in women with a pelvic mass, and to evaluate the prognostic impact of those cytokines. Furthermore, we wanted to examine possible correlations between serum levels of HGF and other cytokines in patients with ovarian epithelial cancer.
2. Materials and methods
2.1. Patients
Blood samples collected from a previously described patient group were applied [5]. The patient group consisted of women diagnosed with a pelvic mass appointed for laparotomy at the Gynecological oncology unit at St. Olavs Hospital in Trondheim, Norway from October 15th 2001 to April 30th 2005. Blood samples were originally collected from 123 women. In the present study, serum was only available from 113 of these women, and of these 57 women had carcinoma, 23 borderline, and 33 benign ovarian tumors. An informed consent was obtained from all participants. Data regarding age at diagnosis and body mass index (BMI) were registered in all cases. Histological type and grade, residual tumor volume at the end of primary surgery, stage of disease according to the International Federation of Gynecology and Obstetrics (FIGO) guidelines [18], and follow-up were registered for the carcinoma group. All histological slides were reviewed by one pathologist (S.H.T), and classified according to the World Health Organization guidelines by histological type and grade of differentiation [19].
2.2. Serum analyses
All serum samples were collected preoperatively. CA 125 was analyzed with immunological methods as part of preoperative routine serum analyses, and the results were extracted from the patients' files. The sera were stored at –80 °C until analyses. No more than two freeze–thaw cycles were allowed for each sample. The serum levels of the following parameters were quantified using the Cytokine Human 25-plex panel (Biosource International, Inc., Camarillo, CA, USA): interleukin (IL)-1β, IL-1 Ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12, IL-13, IL-15, IL-17, tumor necrosis factor (TNF)α, interferon (INF)α, INFγ, granulocyte-macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, MIP-1β, interferon gamma-induced protein (IP)-10, human monokine induced by INFγ (HU-MIG), eotaxin, rantes, and MCP-1. The serum levels of adiponectin, resistin and PAI-I were analyzed by the Human Serum Adipokine panel A kit, and the serum levels of leptin were quantified using the Human Serum Adipokine panel B kit (Linco Research, Inc. St. Charles, MI, USA). The Luminex 100 system (Luminex Corporation, Austin, TX, USA) was applied for the analyses, according to the manufacturers' instructions.
2.3. Statistical analyses
Statistical analyses were performed with the SPSS statistical software program 17.0. For differences in serum cytokine levels between carcinomas, borderline, and benign ovarian tumors, the Kruskal–Wallis test was used. Sub-group analyses were performed with the Mann–Whitney U test. Analysis of correlation between serum cytokines and HGF was performed with the Spearman's rank correlation test.
In the group of carcinomas, comparison of serum cytokine levels between the groups of different histological type and grade was performed with the Kruskal–Wallis test. The Cox proportional hazard regression model was used to evaluate the effect of explanatory variables on overall survival. Five-year overall survival was calculated from the date of primary surgery, to the date of death or status after five years. In the univariate analyses, age, stage, histological type and grade, residual tumor volume, and the cytokines that were elevated in the group of carcinomas were the parameters analyzed. In the analyses of histology, we chose to compare serous and non-serous tumors. The parameters with p-values <0.2 in the univariate analyses were included in a multivariate analysis. In all analyses, p-values <0.05 were considered significant.
3. Results
3.1. Patients characteristics
The mean age at diagnosis was 63.8 years (±11.6 years) in the carcinoma group, 54.8 years (±14.4 years) in the borderline group, and 59.2 years (±13.6 years) in the benign group. There was no significant difference in median BMI between the groups (carcinomas 25.8 (17.0–43.4) kg/m2, borderline 25.1 (20.7–34.4) kg/m2, benign 24.4 (17.5–35.1) kg/m2, p = 0.346). Other characteristics are listed in Table 1.
Table 1.
Characteristics of the cases included (N = 113).
| No. of tumors | % | |
|---|---|---|
| Cancer | 57 | |
| FIGO stage | ||
| I | 19 | 33 |
| II | 3 | 5 |
| III | 29 | 51 |
| IV | 6 | 11 |
| Histological type | ||
| Serous | 28 | 49 |
| Mucinous | 2 | 3 |
| Endometroid | 13 | 23 |
| Clear cell | 6 | 11 |
| Mixed | 6 | 11 |
| Undifferentiated | 2 | 3 |
| Histological grade (G)* | ||
| G1 | 6 | 12 |
| G2 | 12 | 23 |
| G3 | 33 | 65 |
| Borderline | 23 | |
| Serous | 15 | |
| Mucinous | 6 | |
| Clear cell | 1 | |
| Mixed | 1 | |
| Benign | 33 | |
| Epithelial/functional cyst | 20 | 61 |
| Dermoid cyst | 2 | 6 |
| Endometriosis | 1 | 3 |
| Ovarian fibroma | 7 | 21 |
| Uterine myoma | 3 | 9 |
3.2. Serum analyses
The serum levels of CA 125, IL-8, adiponectin, resistin, PAI-1 and leptin (median and range) in the groups of carcinomas, borderline and benign tumors are listed in Fig. 1. Interleukin (IL)-1β, IL-1 Ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-10, IL-12, IL-13, IL-15, IL-17, tumor necrosis factor (TNF)α, interferon (INF)α, INFγ, granulocyte–macrophage colony-stimulating factor (GM-CSF), macrophage inflammatory protein (MIP)-1α, MIP-1β, interferon gamma-induced protein (IP)-10, human monokine induced by INFγ (HU-MIG), eotaxin, rantes, and MCP-1 were detected in less than 80% of the samples and were therefore not included in further analyses. We demonstrate significant differences between carcinomas, borderline tumors, and benign tumors in the serum levels of CA 125, IL-8, and PAI-1 (Fig. 1).
Fig. 1.
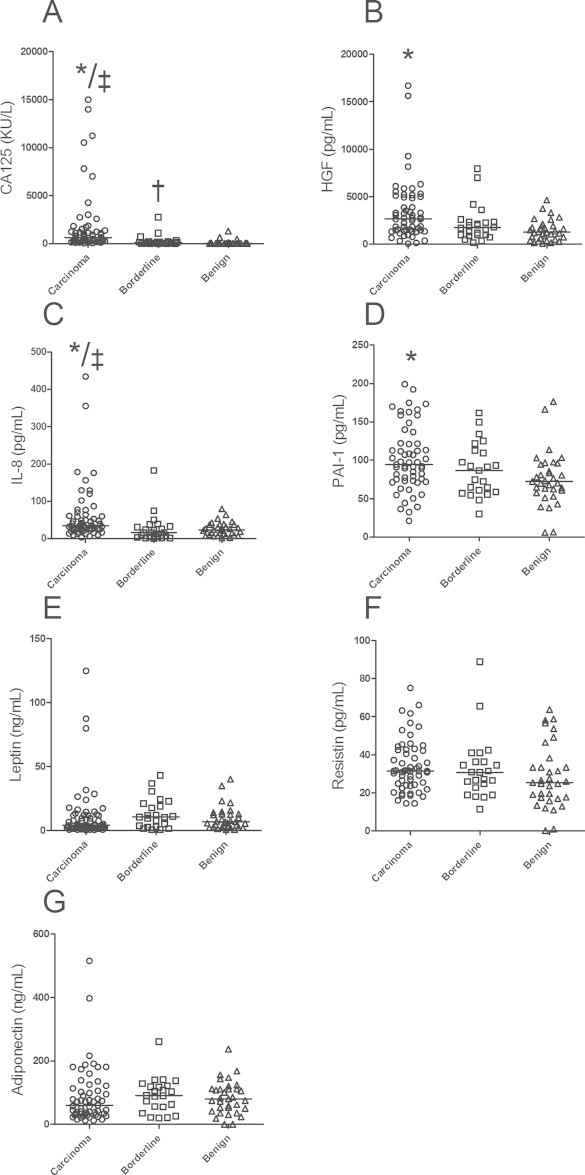
Median serum levels and range of (A) CA 125, (B) HGF, (C) IL-8, (D) PAI-1, (E) leptin, (F) resistin and (G) adiponectin levels in patients with carcinoma, borderline, and benign tumors of the ovary (N = 113). *: comparison carcinomas/benign; ‡: comparison carcinomas/borderline; †: comparison borderline/benign, significant differences, p<0.05.
In separate analyses, including only the carcinomas, there were no differences in serum levels of CA 125, IL-8 or PAI-1 related to histological type or tumor grade. The cases of advanced stage (FIGO stages III and IV) had significantly higher serum levels of CA 125 and IL-8, compared to the cases of early stage cancer (FIGO stages I and II) (median CA125 of 932 vs. 377 KU/L, p = 0.005 and median IL-8 of 40 vs. 30 pg/mL, p = 0.049, respectively).
As mentioned above, we have previously measured serum HGF in the same study population (11). Of the 123 women included in the previous study, sera were only available from 113 women. The median serum HGF level of the 113 women included in the present study was 2717.4 (55–16,689) pg/ml in the carcinoma group, 1668 (177–7955) pg/ml in the borderline group, and 1271 (74–4644) pg/ml in the benign group. We found a significant positive correlation between serum levels of HGF and IL-8 (r = 0.206, p = 0.029), and between HGF and PAI-1 (r = 0.212, p = 0.024). In the analyses of the potential to predict malignancy, the median serum HGF and IL-8 levels in the benign group were chosen as reference values, and cut-off values were determined at 2SD above these values; 3457 pg/ml (1271 + 2186) and 59 pg/ml (23 + 36), respectively. HGF and IL-8 levels below these cut-offs were defined as normal, and levels above were defined as elevated. Of the women with carcinoma, 20 women had elevated HGF levels, and 17 women had elevated IL-8 levels. When we combined the two markers, 30 of the 57 women with carcinoma had either elevated HGF or elevated IL-8 levels.
3.3. Survival
The five-year overall survival for all women with carcinoma was 49%. In the women with early stage cancer, the five-year overall survival was 86%, and in women with advanced stage 26%.
In the univariate analyses of survival, the following parameters were statistically significant: stage of disease, histological type, residual tumor volume, and serum level of CA 125 and IL-8 (Table 2). In a multivariate analysis, age, stage of disease and serum IL-8 level reached statistical significance (Table 3). A Kaplan–Meier plot of 5-year survival in cases with advanced stage ovarian epithelial cancer related to IL-8 level can be seen in Fig. 2.
Table 2.
Univariate analyses of potential prognostic factors for survival in women with ovarian carcinoma (N = 57).
| Variable | HR | 95% CI for HR | p-Value |
|---|---|---|---|
| Early stage | 1 | ||
| Advanced stage | 9.115 | 2.740–30.322 | <0.001 |
| Non-serous histology | 1 | ||
| Serous histology | 3.329 | 1.634–6.738 | 0.01 |
| Grade 1 | 1 | ||
| Grade 2 | 2.077 | 0.431–10.009 | 0.362 |
| Grade 3 | 2.281 | 0.532–9.783 | 0.267 |
| Residual tumor <1 cm | 1 | ||
| Residual tumor ≥1 cm | 6.548 | 2.488–17.421 | <0.001 |
| Age | 1.026 | 0.993–1.060 | 0.123 |
| CA 125 | 1.000 | 1.000–1.000 | 0.030 |
| IL-8 | 1.006 | 1.002–1.010 | 0.007 |
| PAI-1 | 1.003 | 0.994–1.012 | 0.489 |
Table 3.
Multivariate analysis of potential prognostic factors for survival in women with ovarian carcinomas (N = 57).
| Variable | HR | 95% CI for HR | p-Value |
|---|---|---|---|
| Early stage | 1 | ||
| Advanced stage | 7.656 | 1.217–48.177 | 0.030 |
| Non-serous histology | 1 | ||
| Serous histology | 1.264 | 0.524–3.048 | 0.602 |
| Residual tumor <1 cm | 1 | ||
| Residual tumor ≥1 cm | 1.082 | 0.230–5.081 | 0.920 |
| Age | 1.038 | 1.001–1.078 | 0.046 |
| CA 125 | 1.000 | 1.000–1.000 | 0.668 |
| IL-8 | 1.004 | 1.000–1.009 | 0.029 |
Fig. 2.
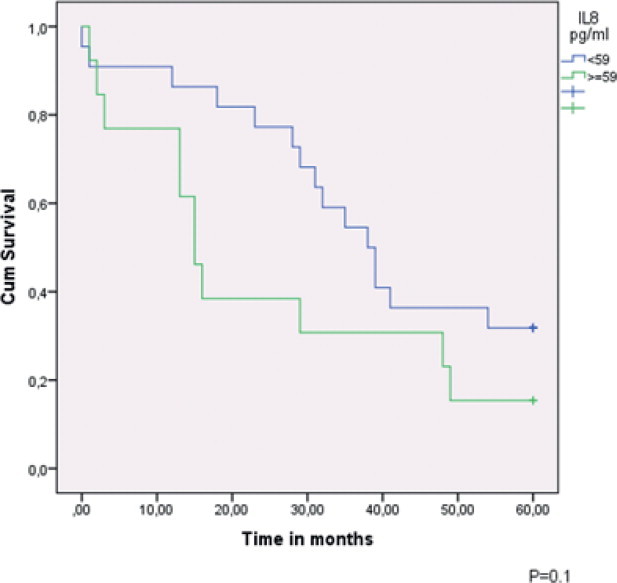
5-year survival in advanced stage epithelial ovarian cancer in cases ≤2SD and >2SD from reference value.
4. Discussion
In the present study we found that the serum levels of CA 125, IL-8, and PAI-1 were significantly higher in women with ovarian epithelial cancer compared to women with benign ovarian tumors.
Most ovarian carcinomas are thought to originate from the surface epithelium or postovulatory inclusion cysts. Damages of the ovarian surface epithelium during ovulation lead to repair processes that attract leukocytes, stimulate release of inflammatory cytokines and nitrous oxide, DNA repair, and tissue restructuring [20]. Repeated ovulations with following repair processes increase the risk of errors in replication, which may cause cancer development [20]. Activation of the nuclear factor κB (NF-κB), a family of signal-activated transcription factors, by proinflammatory cytokines may promote carcinogenesis, and thus represent a link between inflammation and cancer development. NF-κB activation regulates genes that promote tumor cell proliferation, survival, migration, inflammation, and angiogenesis [21].
Elevated serum IL-8 levels in women with ovarian cancer have been reported in several studies [17,22–25]. IL-8 is a CXC-family chemokine, promoting angiogenesis, invasion, and cancer metastasis by binding to the receptors CXCR1 and CXCR2 [25,26]. Induction of IL-8 expression is mainly mediated by NF-κB [26]. We have previously shown HGF to be a marker for ovarian epithelial cancer and an indicator of poor prognosis [5]. By binding to its receptor c-Met, HGF has been reported to enhance NF-κB DNA binding and NF-κB-dependent transcriptional activity [27–29]. Previous studies have reported both decreased and increased cytokine expression in response to HGF. HGF inhibited the NF-κB-mediated proinflammatory cytokine expression in a renal tubular cell line [30]. Furthermore, HGF reduced allergic airway inflammation in asthma by, among other mechanisms, suppressing the T cell cytokine production [31]. On the contrary, HGF enhanced the IL-1-stimulated secretion of IL-8 and MCP-1 in an epithelial colon cell line [32]. Blocking angiogenesis is a novel treatment in platinum resistant ovarian cancer [33]. Both HGF and IL-8 are mediators of angiogenesis. In the present study we found a statistically significant positive correlation between the serum levels of HGF and IL-8, indicating that HGF may be involved in the regulation of IL-8 expression in ovarian epithelial tumors. In analyses of the potential to predict malignancy, we were able to detect more women with carcinoma when both HGF and IL-8 were included, compared to either marker alone.
Some of the markers analyzed in this study (leptin, adiponectin, resistin and PAI-1) are also classified as adipokines. Adipokines are a group of hormones synthesized by adipose tissue, which exert paracrine and endocrine effects in the regulation of metabolism, immunity and inflammation. Adipokines are also involved in human diseases, such as diabetes mellitus and cardiovascular disease, and in processes of angiogenesis and tumor growth. There have been conflicting results regarding the diagnostic value of circulating adipokines in ovarian cancer [34–36]. In the present study, women with ovarian carcinomas had higher serum PAI-1 levels than women with benign ovarian tumors. Higher expression of PAI-1 has been described in ovarian cancer tissue compared to benign ovarian tumor tissue [37], and a prognostic impact of PAI-1 expression in tumor tissue has been described in advanced stage cancers [37]. On the contrary, Abendstein et al. were not able to find a prognostic value of serum PAI-1 levels in women with recurrent ovarian cancer [38]. Little is, however, known about the diagnostic value of serum PAI-1 levels in women with ovarian tumors. Havrilesky et al. described a marker panel, including CA 125, HE4, Glycodelin, Plau-R, MUC-1, and PAI-1 to have a sensitivity of 80.5% and a specificity of 96.5% in predicting early stage ovarian cancer [39].
In the present study, we found no differences in serum levels of adiponectin, leptin, or resistin between women with ovarian carcinoma, borderline, or benign tumors. The adiponectin results are in contrast to the inverse relation between circulating levels of adiponectin and cancer found in several other malignant conditions [40–42]. A diagnostic value of serum leptin was found in a study by Visintin et al., where leptin was included in a panel of six markers [35]. Vrzalova et al. analyzed the same marker panel, but they were not able to replicate the results. Our data are in accordance with Vrzalovas' study, and with a study by Serin et al. [34,36]. The lack of difference in adiponectin and leptin levels between the groups could be explained by the similar BMI.
The five-year overall survival was 49% in the present study, which is in accordance with previous reports [43–45]. In addition to stage of disease, which is a well-documented prognostic factor in ovarian cancer [44], IL-8 had an impact on prognosis in both univariate and multivariate analyses. We found no prognostic impact of serum PAI-1, which is in line with the previous report from women with recurrent ovarian cancer [38]. This result is, however, in contrast to reports of a poor prognosis related to high PAI-1 expression in ovarian cancer tissue [37].
In conclusion, serum levels of IL-8 and PAI-1 were significantly higher in women with ovarian carcinoma compared to women with benign ovarian tumors. Serum levels of IL-8 and PAI-1 were found to correlate positively to serum levels of HGF in women with ovarian tumors in our study. Further studies are needed to evaluate the relation between HGF and IL-8 in ovarian carcinogenesis. This relation could play a role in the blocking of angiogenesis.
References
- 1.Friedlander ML. Prognostic factors in ovarian cancer. Seminars inOncology. 1998;25:305–314. [PubMed] [Google Scholar]
- 2.Jacobs I, Davies AP, Bridges J, Stabile I, Fay T, Lower A. Prevalence screening for ovarian cancer in postmenopausal women by CA 125 measurement and ultrasonography. BMJ. 1993;306:1030–1034. doi: 10.1136/bmj.306.6884.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. International Journal of Cancer. 2000;88:566–574. doi: 10.1002/1097-0215(20001115)88:4<566::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Stany MP, Maxwell GL, Rose GS. Clinical decision making using ovarian cancer risk assessment. American Journal of Roentgenology (AJR) 2010;194:337–342. doi: 10.2214/AJR.09.3669. [DOI] [PubMed] [Google Scholar]
- 5.Aune G, Lian AM, Tingulstad S, Torp SH, Forsmo S, Reseland JE. Increased circulating hepatocyte growth factor (HGF):a marker of epithelial ovarian cancer and an indicator of poor prognosis. Gynecologic Oncology. 2011 doi: 10.1016/j.ygyno.2010.12.355. [DOI] [PubMed] [Google Scholar]
- 6.Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces motility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. Journal of Cell Biology. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanimoto S, Fukumori T, El-Moula G, Shiirevnyamba A, Kinouchi S, Koizumi T. Prognostic significance of serum hepatocyte growth factor in clear cell renal cell carcinoma: comparison with serum vascular endothelial growth factor. Journal of Medical Investigation. 2008;55:106–111. doi: 10.2152/jmi.55.106. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka K, Miki C, Wakuda R, Kobayashi M, Tonouchi H, Kusunoki M. Circulating level of hepatocyte growth factor as a useful tumor marker in patients with early-stage gastric carcinoma. Scandinavian Journal of Gastroenterology. 2004;39:754–760. doi: 10.1080/00365520410005973. [DOI] [PubMed] [Google Scholar]
- 9.Taniguchi T, Kitamura M, Arai K, Iwasaki Y, Yamamoto Y, Igari A. Increase in the circulating level of hepatocyte growth factor in gastric cancer patients. British Journal of Cancer. 1997;75:673–677. doi: 10.1038/bjc.1997.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seidel C, Borset M, Turesson I, Abildgaard N, Sundan A, Waage A. Elevated serum concentrations of hepatocyte growth factor in patients with multiple myeloma. The Nordic Myeloma Study Group. Blood. 1998;91:806–812. [PubMed] [Google Scholar]
- 11.Ness RB, Cottreau C. Possible role of ovarian epithelial inflammation in ovarian cancer. Journal of the National Cancer Institute. 1999;91:1459–1467. doi: 10.1093/jnci/91.17.1459. [DOI] [PubMed] [Google Scholar]
- 12.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clinical Cancer Research. 2007;13:2385–2391. doi: 10.1158/1078-0432.CCR-06-1828. [DOI] [PubMed] [Google Scholar]
- 13.Scambia G, Testa U, Panici PB, Martucci R, Foti E, Petrini M. Interleukin-6 serum levels in patients with gynecological tumors. International Journal of Cancer. 1994;57:318–323. doi: 10.1002/ijc.2910570305. [DOI] [PubMed] [Google Scholar]
- 14.Uslu R, Sanli UA, Dikmen Y, Karabulut B, Ozsaran A, Sezgin C. Predictive value of serum interleukin-8 levels in ovarian cancer patients treated with paclitaxel-containing regimens. International Journal of Gynecological Cancer. 2005;15:240–245. doi: 10.1111/j.1525-1438.2005.15210.x. [DOI] [PubMed] [Google Scholar]
- 15.Clendenen TV, Lundin E, Zeleniuch-Jacquotte A, Koenig KL, Berrino F, Lukanova A. Circulating inflammation markers and risk of epithelial ovarian cancer. Cancer Epidemiology, Biomarkersand Prevention. 2011;20:799–810. doi: 10.1158/1055-9965.EPI-10-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sasaroli D, Coukos G, Scholler N. Beyond CA125: the coming of age of ovarian cancer biomarkers. Are we there yet? Biomarkers in Medicine. 2009;3:275–288. doi: 10.2217/bmm.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorelik E, Landsittel DP, Marrangoni AM, Modugno F, Velikokhatnaya L, Winans MT. Multiplexed immunobead-based cytokine profiling for early detection of ovarian cancer. Cancer Epidemiology, Biomarkersand Prevention. 2005;14:981–987. doi: 10.1158/1055-9965.EPI-04-0404. [DOI] [PubMed] [Google Scholar]
- 18.Benedet JL, Bender H, Jones 3rd H, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. International Journal of Gynecologyand Obstetrics. 2000;70:209–262. [PubMed] [Google Scholar]
- 19.Tavassoli FA, Devilee P. International Agency for Research on Cancer. Lyon; 2003. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. [Google Scholar]
- 20.White KL, Vierkant RA, Phelan CM, Fridley BL, Anderson S, Knutson KL. Polymorphisms in NF-kappaB inhibitors and risk of epithelial ovarian cancer. BMC Cancer. 2009;9:170. doi: 10.1186/1471-2407-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Waes C, Yu M, Nottingham L, Karin M. Inhibitor-kappaB kinase in tumor promotion and suppression during progression of squamous cell carcinoma. Clinical Cancer Research. 2007;13:4956–4959. doi: 10.1158/1078-0432.CCR-07-1287. [DOI] [PubMed] [Google Scholar]
- 22.Edgell T, Martin-Roussety G, Barker G, Autelitano DJ, Allen D, Grant P. Phase II biomarker trial of a multimarker diagnostic for ovarian cancer. Journal of Cancer Research and Clinical Oncology. 2010;136:1079–1088. doi: 10.1007/s00432-009-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nolen B, Marrangoni A, Velikokhatnaya L, Prosser D, Winans M, Gorelik E. A serum based analysis of ovarian epithelial tumorigenesis. Gynecologic Oncology. 2009;112:47–54. doi: 10.1016/j.ygyno.2008.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai-Turton M, Santillan A, Lu D, Bristow RE, Chan KC, Shih Ie M. p53 autoantibodies, cytokine levels and ovarian carcinogenesis. Gynecologic Oncology. 2009;114:12–17. doi: 10.1016/j.ygyno.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lokshin AE, Winans M, Landsittel D, Marrangoni AM, Velikokhatnaya L, Modugno F. Circulating IL-8 and anti-IL-8 autoantibody in patients with ovarian cancer. Gynecologic Oncology. 2006;102:244–251. doi: 10.1016/j.ygyno.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY. Effect of interleukin-8 gene silencing with liposome-encapsulated small interfering RNA on ovarian cancer cell growth. Journal of theNational Cancer Institute. 2008;100:359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown M, Cohen J, Arun P, Chen Z, Van Waes C. NF-kappaB in carcinoma therapy and prevention. Expert Opinion on Therapeutic Targets. 2008;12:1109–1122. doi: 10.1517/14728222.12.9.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan S, Gao M, Meng Q, Laterra JJ, Symons MH, Coniglio S. Role of NF-kappaB signaling in hepatocyte growth factor/scatter factor-mediated cell protection. Oncogene. 2005;24:1749–1766. doi: 10.1038/sj.onc.1208327. [DOI] [PubMed] [Google Scholar]
- 29.Muller M, Morotti A, Ponzetto C. Activation of NF-kappaB is essential for hepatocyte growth factor-mediated proliferation and tubulogenesis. Molecular and Cellular Biology. 2002;22:1060–1072. doi: 10.1128/MCB.22.4.1060-1072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannopoulou M, Dai C, Tan X, Wen X, Michalopoulos GK, Liu Y. Hepatocyte growth factor exerts its anti-inflammatory action by disrupting nuclear factor-kappaB signaling. American Journal of Pathology. 2008;173:30–41. doi: 10.2353/ajpath.2008.070583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito W, Takeda M, Tanabe M, Kihara J, Kato H, Chiba T. Anti-allergic inflammatory effects of hepatocyte growth factor. International Archives of Allergy and Immunology. 2008;146(Suppl. 1):82–87. doi: 10.1159/000126067. [DOI] [PubMed] [Google Scholar]
- 32.Grygas J, Steiger N, LeSeur CL, Unger BL, McGee DW. Hepatocyte growth factor enhances IL-1beta stimulated IL-8 secretion by Caco-2 epithelial cells. In Vitro Cellularand Developmental Biology-Animal. 2007;43:147–152. doi: 10.1007/s11626-007-9018-4. [DOI] [PubMed] [Google Scholar]
- 33.Perren TJ, Swart AM, Pfisterer J, Ledermann JA, Pujade-Lauraine E, Kristensen G. A phase 3 trial of bevacizumab in ovarian cancer. New England Journal of Medicine. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 34.Serin IS, Tanriverdi F, Yilmaz MO, Ozcelik B, Unluhizarci K. Serum insulin-like growth factor (IGF)-I, IGF binding protein (IGFBP)-3, leptin concentrations and insulin resistance in benign and malignant epithelial ovarian tumors in postmenopausal women. Gynecological Endocrinology. 2008;24:117–121. doi: 10.1080/09513590801895559. [DOI] [PubMed] [Google Scholar]
- 35.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y. Diagnostic markers for early detection of ovarian cancer. Clinical Cancer Research. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 36.Vrzalova J, Prazakova M, Novotny Z, Topolcan O, Casova M, Holubec Jr L. Test of ovarian cancer multiplex xMAP technology panel. Anticancer Research. 2009;29:573–576. [PubMed] [Google Scholar]
- 37.Kuhn W, Schmalfeldt B, Reuning U, Pache L, Berger U, Ulm K. Prognostic significance of urokinase (uPA) and its inhibitor PAI-1 for survival in advanced ovarian carcinoma stage FIGO IIIc. British Journal of Cancer. 1999;79:1746–1751. doi: 10.1038/sj.bjc.6690278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abendstein B, Daxenbichler G, Windbichler G, Zeimet AG, Geurts A, Sweep F. Predictive value of uPA, PAI-1, HER-2 and VEGF in the serum of ovarian cancer patients. Anticancer Research. 2000;20:569–572. [PubMed] [Google Scholar]
- 39.Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecologic Oncology. 2008;110:374–382. doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 40.Chen DC, Chung YF, Yeh YT, Chaung HC, Kuo FC, Fu OY. Serum adiponectin and leptin levels in Taiwanese breast cancer patients. Cancer Letters. 2006;237:109–114. doi: 10.1016/j.canlet.2005.05.047. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clinical Cancer Research. 2005;11:466–472. [PubMed] [Google Scholar]
- 42.Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73:261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]
- 43.Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. European Journal of Cancer. 2009;45:931–991. doi: 10.1016/j.ejca.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 44.Colombo N, Van Gorp T, Parma G, Amant F, Gatta G, Sessa C. Ovarian cancer. Critical Reviews in Oncology. 2006;60:159–179. doi: 10.1016/j.critrevonc.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Cancer Registry of Norway. Cancer in Norway 2008—cancer incidence, mortality, survival and prevalence in Norway. Oslo: Cancer registry of Norway; 2009.


