Table 2.
[2 + 2] Dimerization of aromatic alkenes via photoinduced electron transfera
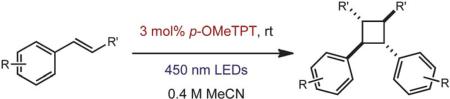
| ||||
|---|---|---|---|---|
| Entry | Substrate | Electron relay | Product | Yieldb,c |
| 1 |
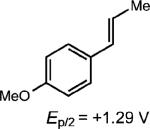
|
0.5 equiv. naphthalene |
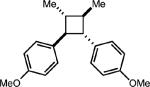
|
54% (0%) |
| 2 |
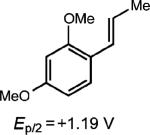
|
0.25 equiv. anthracene |
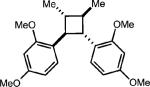
|
46% (3%) |
| 3 |
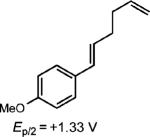
|
0.5 equiv. naphthalene |
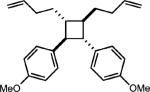
|
51% (30%) |
| 4 |
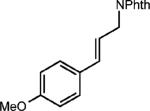
|
0.5 equiv. naphthalene |
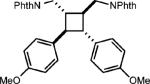
|
42% (27%) |
| 5d |
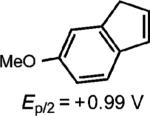
|
3 equiv. propylene oxide |
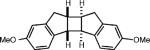
|
72% (0%) |
| 6e |
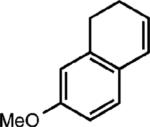
|
None |
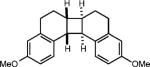
|
(62%) |
| 7 |
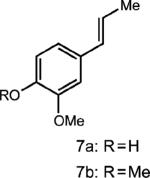
|
0.25 equiv. anthracene |
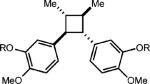
|
0% (0%) |
| 8 |
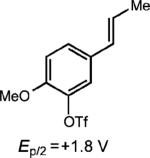
|
None |
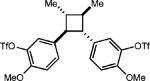
|
(31%) |
| 9f |
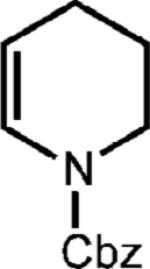
|
0.25 equiv. naphthalene |
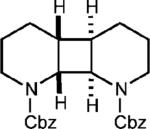
|
78% (14%) |
Reactions carried out in 0.4 M degassed acetonitrile.
Average of two isolated yields on a 100 mg scale.
Parenthetical product yield in absence of electron relay.
Reaction carried out at 0 °C in sparged dichloromethane.
Reaction carried out at –10 °C in sparged dichloromethane.
Reaction carried out at –10 °C in acetonitrile.
