Abstract
Background
Corneal scars are commonly formed following many diseases of the eye like trauma, inflammation and infections. They lead to permanent diminution of vision which can be managed by Penetrating Keratoplasty (PK). PK is removing diseased as well as healthy tissues and is associated with many post-operative complications. Deep Anterior Lamellar Keratoplasty (DALK) is a relatively newer procedure which replaces only the diseased stroma, leaving the original corneal endothelium intact. This procedure is associated with lesser incidence of post-operative complications.
Methods
The study was conducted at a large tertiary care centre. 10 patients with stromal corneal scars were subjected to DALK and results were analysed after 06 months. Poor quality donor corneal tissue of B− and C grade was used in all cases.
Results
7 out of 10 patients (70%) undergoing DALK had post-operative visual acuity of 6/24 or better. 03 patients who did not have adequate recovery of visual acuity were due to over-riding of the graft in 01 case (10%), fungal keratitis in 01 case (10%) and interface haze in 01 case (10%).
Conclusion
DALK is a promising new technique for management of superficial corneal stromal scars using poor quality donor corneal tissue. Initial results are encouraging with minimal complications.
Keywords: Lamellar keratoplasty, Superficial stromal corneal scars, Descemet's perforation, Graft rejection, Interface haze
Introduction
Full-thickness penetrating keratoplasty (PK) has been the preferred therapy of visual loss due to scarring following corneal diseases.1 The technique of PK has been modified in the past 25 years owing to advances in surgical techniques, medications and storage of donor material. However, PK is an indiscriminate form of surgery, it replaces all the layers of cornea, regardless of the layer that is responsible for visual deficit. In cases of anterior stromal scars with healthy endothelium, PK removes the scar but also sacrifices the healthy endothelium as collateral damage.
Anterior Lamellar Keratoplasty is a surgical procedure in which the anterior layers of the cornea (epithelium, basement membrane, Bowman's layer and stroma) to a variable depth are excised and replaced by donor corneal tissue. Compared with a penetrating keratoplasty, lamellar procedure has the advantage of avoiding most complications associated with “open sky” surgery, easier post-operative management and less risk of allograft rejection.2 Despite these benefits, surgeons routinely perform a penetrating keratoplasty for anterior corneal disorders because the latter technique is easier to perform and lamellar transplants often show decreased best-corrected visual acuity owing irregular astigmatism and/or scarring at the donor to recipient interface. Less scarring may occur with use of “big bubble”, hydro-delamination or photoablation of the posterior stroma have been advocated to obtain a smooth recipient stromal bed.3–5
Deep Anterior Lamellar Keratoplasty (DALK) is a logical step in the surgical management of corneal stromal opacification with a functional, healthy endothelium. In DALK, pathological stroma is excised down to Descemets membrane (DM) and offers the promise of better visual outcome as compared to conventional lamellar grafting.6
A study was conducted at a large referral hospital to clinically evaluate the following:
-
1.
Assessment of final visual outcome following DALK in corneal stromal disorders.
-
2.
To evaluate intra-operative and post-operative complications of DALK.
Materials and methods
Patients requiring keratoplasty for superficial corneal stromal opacities without endothelial disease between June 2008 and June 2009 in a large tertiary care hospital were enrolled into the study. All patients received detailed explanation about the purpose of the study as well as advantages and disadvantages of DALK. Informed written consent was obtained from all patients who agreed to participate in the study. Eyes that required additional surgery such as cataract extraction with keratoplasty were not included in the study. Surgery was performed by two surgeons with a large experience in PK. 13 eyes of 13 patients were assigned to undergo DALK. Intra-operative descemets membrane perforation occurred in 03 eyes, DALK was abandoned in these and they were converted into PK and not included in this study. These 10 (ten) eyes were followed up for a period of 6 months to note the results.
Exclusion criteria: patients with any posterior segment anomaly, secondary glaucoma and derangement of anterior segment were excluded for DALK.
Procurement of donor tissue
Donor tissue procurement was through hospital cornea retrieval programme. Blood samples for serology to rule out HIV and Hepatitis B infection were sent in all cases. Storage of the donor tissue was done in the McCarey-Kaufman (MK) media. Death to refrigeration time was kept below 6 h and no donor tissue was used after storage for more than 48 h.
Donor tissue found acceptable after evaluation was used for DALK after confirming that the serological tests for HIV and Hepatitis B were negative. Donor tissue was graded as per Orbis guidelines. Grade B or poorer donor corneas were used for performing DALK in our study (Table 1).
Table 1.
Clinical evaluation of donor corneal material (based on Orbis telemedicine guidelines).
| Grade A | Mild epithelial oedema present. Thickness of cornea increased by 10%. |
| Grade B+ | Mild epithelial oedema present. Descemets folds in periphery present. Peripheral endothelial changes present. Corneal thickness increased by 10–25%. Changes disappear after increasing IOP or by temperature reversal. |
| Grade B | Moderate epithelial oedema present. Mild stromal oedema present. Moderate descemets folds present. Mild endothelial changes present in the centre. Increase in corneal thickness by 25–30%. |
| Grade B− | Severe epithelial oedema with denudation of epithelium at places. Epithelium can be removed easily. Moderate stromal oedema. Descemets folds some of which are reaching the centre. Increase in thickness by 30–50%. |
| Grade C | All above changes in moderate to severe degree. Increase in thickness by 50–100%. |
| Grade D | Cornea is totally hazy and details of anterior chamber and other structures not seen. |
Preparation of recipient cornea
Anwar's big bubble technique
Trephination is performed using a manual trephine upto 60–80% of stromal thickness. A 26-gauge needle is attached to an air filled syringe. The needle is bent about 5 mm from its tip to an angle of 60° with bevel facing up to minimise inadvertent descemets perforation. The tip is inserted bevel up deep into the corneal stroma just above the descemets membrane at a site in the trephination groove where clear area of cornea is present. The needle is advanced into the partially cut central button for 3–4 mm from its entry point. At this point, 0.5–0.75 ml of air is injected leading to formation of a large air bubble with a circular outline between descemets and deepest stroma. This is the desired result and is indicated by the sudden easing of resistance of the plunger, as well as the “explosive” appearance of a whitish, semi-opaque disc the edge of which may coincide with the trephination groove.
Once a big bubble has been achieved a paracentesis is performed – this enables the descemets membrane to fall away from the corneal stroma. A partial thickness anterior keratectomy is carried out by means of dissection with a bard parker blade or a disposable knife, leaving a layer of corneal stroma in place anterior to the bubble. Using a sharp-tipped blade (such as 15° side port knife) small nick is made through the remaining stromal layers and remainder of deep stroma dissected out leaving descemets membrane bare.
Preparation of donor tissue
Endothelium and DM from the donor button are removed to preserve a smooth posterior stromal surface. The donor graft of appropriate size (6.5–7.5 mm) was first cut using a punch. Leaving DM behind may cause interface opacities and wrinkles. Stromal rim is probed with a dry weck cel sponge for creating a small detachment at the outer edge of the graft. The DM and the endothelium can now be stripped using a forceps and sharp disposable knife off in one piece. The anterior stromal graft was sutured to the previously prepared recipient corneal bed using 10/0 nylon sutures.
Post-operative follow-up
All patients were followed up for 01 year and following findings were noted.
-
1.
Visual acuity: best-corrected visual acuity was noted after 02 months of surgery.
-
2.
Slit lamp examination: for signs of graft rejection, inflammation, uveitis, neovascularisation and wound integrity.
-
3.
Graft clarity/interface haze
-
4.
Intraocular pressure: measured by non-contact or schiotz tonometer.
-
5.
Endothelial count following DALK using specular microscope.
The sutures were removed any time after 12 weeks if they were seen to contribute to graft neovascularisation and caused a significant amount of foreign body sensation and lacrimation.
Results
The results were compiled and analysed at the end of the study.
Our study included 10 patients, 05 of them being male and 05 females. Distribution of patients as per age is shown in Table 2. The most common indication for DALK was post-herpetic keratitis in 4 (40%) cases (Fig. 1). This was followed by post-microbial keratitis corneal scarring in 02 (20%) cases, post-traumatic corneal scarring 2 (20%) cases and 02 (20%) cases of corneal dystrophy (Granular & Salzmann) (Fig. 2).
Table 2.
Age distribution of patients for DALK.
| Age | No. of patients |
|---|---|
| 21–30 | 02 |
| 31–40 | 02 |
| 41–50 | – |
| 51–60 | 01 |
| 61–70 | 03 |
| 71–80 | 02 |
Fig. 1.
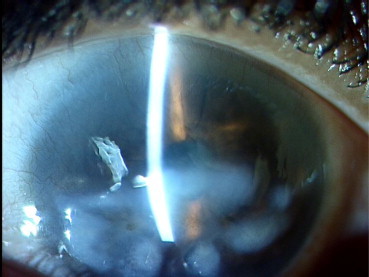
Superficial corneal stromal scar following herpetic keratitis.
Fig. 2.
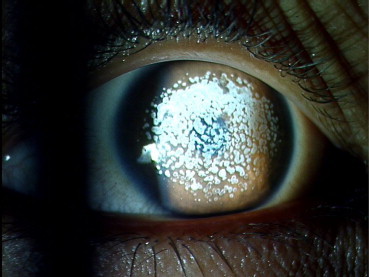
Salzmann nodular degeneration.
In the intra-operative period, 03 out of initial 13 cases (23.07%) had perforation of DM leading to conversion into PK, these cases were then excluded from the study.
Amongst the post-operative complications, 01 case had poor graft–host apposition.
01 case (10%) had persistent severe graft–host interface haze (Fig. 3). This did not resolve and PK had to be performed 06 months later (Table 3).
Fig. 3.
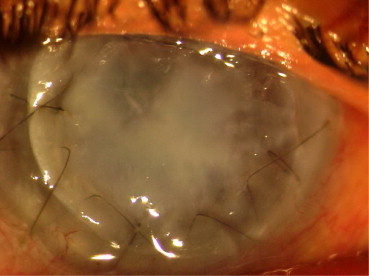
Severe interface haze following DALK.
Table 3.
Post-operative complications following DALK.
| Complication | No |
|---|---|
| Graft–host interface haze | 1 |
| Poor graft–host apposition | 1 |
| Recurrent viral stromal keratitis | 1 |
| Infection (fungal) | 1 |
| Graft rejection | – |
| PED | – |
| Primary graft failure | – |
| Graft ectasia | 1 |
At 04 weeks of post-operative follow-up 01 (10%) of the remaining cases of DALK developed recurrent viral stromal keratitis (Fig. 4) and 01 (10%) case developed fungal keratitis in which fungal hyphae was confirmed by laboratory (Fig. 5). Though infection was controlled by the use of topical medications, the patient could not gain useful vision due to opacification of the graft. Late follow-up at 06 months showed development of graft ectasia in 01 (10%) case (Fig. 6, Table 4).
Fig. 4.
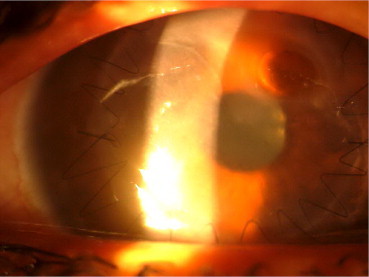
Recurrence of herpetic keratitis following DALK.
Fig. 5.
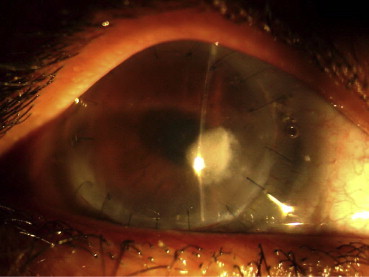
Fungal keratitis following DALK.
Fig. 6.
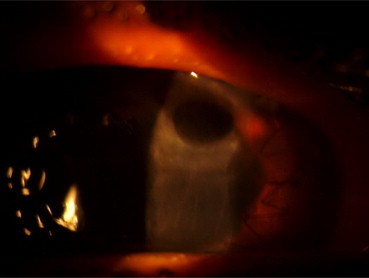
Graft ectasia formation following DALK.
Table 4.
Final BCVA attained in relation to quality of donor material used.
| Ser. No. | Donor tissue grade | Final BCVA |
|---|---|---|
| 1 | B | 6/18 |
| 2 | B− | CFCF |
| 3 | B− | 6/9 |
| 4 | B | 6/6 |
| 5 | C | 6/24 |
| 6 | B− | 1/60 |
| 7 | B− | 1/60 |
| 8 | B | 6/12 |
| 9 | C | 6/24 |
| 10 | B− | 6/12 |
7 out of 10 (70%) patients eventually attained BCVA of 6/24 or better which is excellent after corneal disorders. Recovery of BCVA and endothelial counts following the procedure are shown in Tables 5 and 6 respectively. It was possible to take these readings only in grafts which remained clear (Fig. 7).
Table 5.
Recovery of BCVA following DALK.
| Follow-up period | Mean visual acuity (numeric scale) | Standard deviation | T | Sig (2-tailed) |
|---|---|---|---|---|
| Day 1 | 0.175 | 0.198 | 1.717 | 0.103 |
| Day 7 | 0.195 | 0.189 | 1.472 | 0.158 |
| Day 30 | 0.333 | 0.240 | 2.998 | 0.008 |
| Day 90 | 0.344 | 0.324 | 1.989 | 0.062 |
| Day 180 | 0.353 | 0.320 | 1.748 | 0.048 |
Table 6.
Mean endothelial density of DALK at various follow-up periods and its statistical significance.
| Follow-up period | Mean endothelial count (per sq mm) | Standard deviation | T | Sig (2-tailed) |
|---|---|---|---|---|
| Day 1 | 2203 | ±157 | 1.251 | 0.227 (NS) |
| Day 30 | 2072 | ±177 | 2.387 | 0.028 (Sig) |
| Day 90 | 1983 | ±174 | 1.673 | 0.112 (NS) |
| Day 180 | 1985 | ±170 | 3.144 | 0.006 (Sig) |
Sample size (N) = 10, NS = Not Significant, 0.05 = Significant at 0.05 level (confidence limit 95%), 0.01 = Significant at 0.01 level (confidence limit 99%).
Fig. 7.
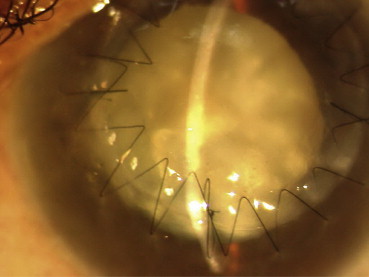
Clear graft with cataract following DALK.
Discussion
Replacement of diseased tissue with healthy donor cornea in DALK can result in improvement of visual acuity whereas risk of endothelial rejection or intraocular complications can be reduced.7 In addition, more donor corneas can be utilised since the procedure does not require a healthy donor endothelium. This is an important issue in countries where good donor corneas are difficult to get.
In cases of superficial corneal opacities, for which DALK is indicated, examination of endothelial cell counts generally is unavailable preoperatively. Specular microscopy 6 months after DALK revealed that over 60% of the eyes had endothelial cell counts of more than 2000/sq mm. It should be noted, that DALK is not free of endothelial damage.8 Even in uncomplicated cases, upto 20% loss of endothelial cell density was noted at 06 months. This is could be a result of surgical manipulations being performed immediately above the DM.9This indicates that DALK should not be performed in eyes with decreased endothelial density. Inadvertent penetration of the DM is not an uncommon complication with the incidence reported between 0 and 39.2%.10 Corneal endothelial density stabilised after 03 months. We performed cataract surgery in two eyes after DALK and no considerable endothelial loss was noted post-operatively. Although we cannot draw a conclusion with a small sample size, cataract surgery following DALK may cause fewer complications such as endothelial damage or inciting an immunologic rejection reaction compared with PK.
Rise in intraocular pressure was seen more often after PK than DALK. This is probably due to prolonged use of topical corticosteroids after PK. In DALK we used corticosteroid eye drops for approximately 4 months, after which all medications other than lubricants were discontinued. The early discontinuation of corticosteroid eye drops may also be beneficial for decreased risk of infection and cataract development. None of the eyes in DALK group suffered immunologic rejection, though this is anticipated to occur in approximately 20–30% of eyes after PK.11,12
Panda and associates reported results of a nonrandomized prospective study comparing DALK and PK.13 The authors reported that DALK group showed better visual acuity, higher rate of clear grafts, less astigmatism and better endothelial density. Uncomplicated DALK has a number of advantages of being an extra-ocular procedure. There are no post-operative intraocular complications such as formation of anterior synechiae or secondary glaucoma. The graft–host apposition is very good and incidences of over-riding/under-riding at the interface are minimal leading to a quicker visual recovery and a more stable wound.10
Experiments with DALK suggested that it was possible to obtain a marked improvement in post-operative visual acuity. Pathological corneal stroma was completely excised till the level of DM, particularly in the papillary axis.14 We performed this procedure in wide variety of clinical conditions affecting the corneal stroma varying from post-infectious scars to corneal stromal dystrophies.15 Descemets membrane is a basement membrane of uniform structure, whose thickness is thought to be increasing with age. Histologically, it is 10–13 micron thick in adults there have been very few reports on techniques that expose DM intentionally during surgery. However if effort is made to expose DM during surgery, an area about 5 mm diameter in the pupillary zone, it is found that there are no adhesions between the DM and the stroma.
The endothelial side of the donor cornea is stripped clear of the DM and endothelium using forceps and a crescent knife, leaving a smooth surface on the stromal side. The result, no interface haze occurs between the host cornea where DM is present and the donor corneal stromal button from which DM has been removed. This is probably the reason there is significant improvement in visual acuity, 06 months from date of the procedure.16
In our study, there was no endothelial rejection amongst 10 eyes, post-DALK. Following DALK, patient's own endothelial cells are active, there is no danger of an endothelial rejection reaction and the graft–host interface is very stable.
Conclusions
In conclusion, DALK seems to offer several advantages over PK. It is essentially an extra-ocular procedure and avoids potential intraocular damage that is associated with PK. Visual results are better than those of PK. The endothelial cell loss is generally small; topical steroids are needed for shorter periods, allowing less steroid inhibition of healing of the corneal interface. In turn, this means that suture removal may be undertaken with confidence at a much earlier date with quicker rehabilitation and lesser incidence of graft rejection.
As a bonus, we have got good visual outcomes even with bad quality grafts (grade B− and C). These would have certainly failed had PK been performed.
According to our experience, DALK should be strongly recommended for high-risk cases such as eyes with neovascularisation and those with superficial to mid-stromal pathology and healthy endothelium.
Conflicts of interest
All authors have none to declare.
References
- 1.Zirm E. Eine crfolgreiche totalc Keratoplastik. Arch Ophthalmol. 1906;64:580–591. [Google Scholar]
- 2.Kondo J., Sugita J. Deep lamellar keratoplasty. J Eye (Atarasiiganka) 1992;9:2061–2064. [Google Scholar]
- 3.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. in press;28. [DOI] [PubMed]
- 4.Sugita J., Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anwar M. Dissection technique in lamellar keratoplasty. Br J Ophthalmol. 1972;56:711–713. doi: 10.1136/bjo.56.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shimazaki J., Shimmura S., Tsubota K. Randomized clinical trial of deep lamellar keratoplasty vs penetrating keratoplasty. Am J Ophthalmol. 2002;134:159–165. doi: 10.1016/s0002-9394(02)01523-4. [DOI] [PubMed] [Google Scholar]
- 7.Melles G.R.J., Lander F., Rietveld F.J.R. A new surgical technique for deep stromal, anterior lamellar keratoplasty. Br J Ophthalmol. 1999;83:327–333. doi: 10.1136/bjo.83.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olson T. Postop changes in endothelial cell density of corneal grafts. Acta Ophthalmol. 1985;59:863–870. [Google Scholar]
- 9.Morris E., Kirwon J., Sujatha S., Rostran C. Corneal endothelial specular microscopy following deep lamellar keratoplasty with lyophilized tissue. Eye. 1998;12:612–622. doi: 10.1038/eye.1998.155. [DOI] [PubMed] [Google Scholar]
- 10.Fogla R., Padmanabhan Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006 Feb;141(2):254–259. doi: 10.1016/j.ajo.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 11.Price F.W., Whitson W.E., Collins K.S. Five-year corneal graft survival: a large, single-centre patient cohort. Arch Ophthalmol. 1993;111:799–805. doi: 10.1001/archopht.1993.01090060087029. [DOI] [PubMed] [Google Scholar]
- 12.Thompson R.W., Price O.M., Bowers P.J., Price F.W., Jr. Long term graft survival after penetrating keratoplasty. Ophthalmology. 2003;110:1396–1402. doi: 10.1016/S0161-6420(03)00463-9. [DOI] [PubMed] [Google Scholar]
- 13.Panda A., Bageshwar L.M.S., Ray M., Singh J.P., Kumar A. Deep lamellar keratoplasty versus penetrating keratoplasty for corneal lesions. Cornea. 1999;18:172–175. doi: 10.1097/00003226-199903000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Parthasarathy A., Por Y.M., Tan D.T. Use of a ‘small-bubble technique’ to increase the success of Anwar's ‘big-bubble technique’ for deep lamellar keratoplasty with complete baring of Descemet's membrane. Br J Ophthalmol. 2007 Oct;91(10):1369–1373. doi: 10.1136/bjo.2006.113357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimmura S., Tsubota K. Deep anterior lamellar keratoplasty. Curr Opin Ophthalmol. 2006 Aug;17(4):349–355. doi: 10.1097/01.icu.0000233953.09595.91. [DOI] [PubMed] [Google Scholar]
- 16.Marchini G., Mastropasqua L., Pedrotti E., Nubile M., Ciancaglini M., Sbabo A. Deep lamellar keratoplasty by intracorneal dissection: a prospective clinical and confocal microscopic study. Ophthalmology. 2006 Aug;113(8):1289–1300. doi: 10.1016/j.ophtha.2006.01.071. [DOI] [PubMed] [Google Scholar]


