Table 3.
Chemical structures of treatment options for D. fragilis infections.
| Treatment options | Chemical formula | Molecular structure | Side effects/contraindications | Recommended dosagea |
|---|---|---|---|---|
| Carbarsone | C7H9AsN2O4 | Long-term exposure to As has been associated with bladder and kidney cancer; contraindicated for patients with severe hepatic disease | 75 mg/kg × 10 days | |
| Diphetarsone | C14H18As2N2O6 | 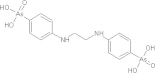 |
Transient hepatic abnormalities, contraindicated for patients with severe hepatic disease | 500 mg POb tidc × 10 days |
| Tetracycline | C22H24N2O8 | 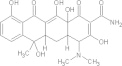 |
Detrimental effects on dental development/contraindicated for children under the age of 5 yrs old, renal impaired patients and during pregnancy | 500 mg PO qidd × 10 days |
| 40 mg/kg/day (max. 2 g) PO qid × 10 days | ||||
| Oxytetracycline | C22H24N2O9 | 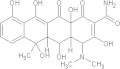 |
Discolouration of teeth; affects foetal skeletal development/contraindicated for children under 8 yrs old, renal impaired and during pregnancy | General: 250–500 mg PO qid × 10 days |
| For severe infections: 250–500 mg PO qid × 7–30 days | ||||
| Doxycycline | C22H24N2O8 | 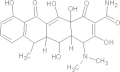 |
Gastrointestinal disturbances; esophageal ulceration; photosensitising agent/contraindicated for children under the age of 8 yrs old, renal impaired patients and during pregnancy | 100–200 mg PO qde × 7–14 days |
| 1–2 mg/kg/day PO tid | ||||
| Iodoquinol (hydroxyquinoline) | C5H5I2NO | 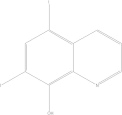 |
Nausea; vomiting; abdominal cramps; diarrhoea; skin irritation; fever; chills; headache; dizziness | 650 mg PO tid × 7–10 days |
| 30–40 mg/kg/day PO in three doses × 7–10 days | ||||
| Clioquinol | C9H5CIINO | 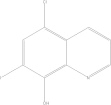 |
Subacute Myelo-Optico-Neuropathy (SMON) | 250 mg PO tid × 7 days |
| 40 mg/kg/day PO × 10 days | ||||
| Erythromycin | C37H67NO13 | 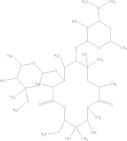 |
GI disturbances; arrhythmia; neurological reactions; contraindicated for women during pregnancy | 500 mg PO bidf × 10–14 days |
| 30–100 mg/kg/day × 10–14 days | ||||
| Paromomycin | C23H47N5O18S. H2SO4 | 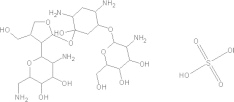 |
Diarrhoea; nausea; stomach cramps/seek medical attention for severe allergic reactions | 25–35 mg/kg/day PO tid × 7 daysg |
| Metronidazole | C6H9N3O3 | 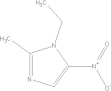 |
Loss of appetite; metallic taste; headache; insomnia; vertigo; anorexia; vomiting/seek medical attention if experiencing severe adverse effects | 500–750 mg PO tid × 10 days |
| 35–50 mg/kg/day PO tid × 10 days | ||||
| Secnidazole | C7H11N3O3 | 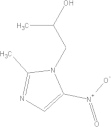 |
GI disturbances; nausea | 2 g qd PO |
| 30 mg/kg qd PO | ||||
| Ornidazole | C7H10CIN33 | 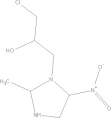 |
GI disturbances; nausea; headache; dizziness | 2 g qd PO |
| 30 mg/kg qd PO | ||||
| Tinidazole | C8H13N3O4S | 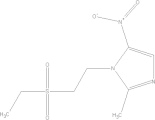 |
GI disturbances; bitter taste; diarrhea; itchiness | 2 gg qd PO × 3 days |
| 50 mg/kg qd × 3 days |
Adult and paediatric dosages shown where applicable, paediatric dosage is shown below adult dosage.
PO = Perorally.
“tid” = “ter in die”; “three times a day”.
“qid” = “quater in die”; “four times a day”.
“qd” = “quaque die”; “once a day”.
“bid” = “bis in die”; “twice a day”.
Universal dosage for adults and children.
