Fig. 2.
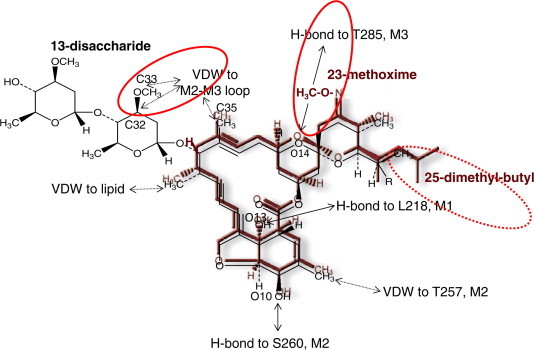
Interaction of ivermectin (IVM) with a glutamate-gated chloride channel (GluCl) as proposed by Hibbs and Gouaux (2011), showing moxidectin (MOX; maroon) superimposed over IVM (black). Note that while some of the interaction sites are shared in common between MOX and IVM (O10, O13, C18, C35 and C48), other interaction sites of IVM with the GluCl are either absent (C32, C33; both forming van der Waal (VDW) interactions with the GluCl in the case of IVM) due to the absence of any saccharide group in MOX, or are blocked/altered (O14; H-bond in the case of IVM) by the C23 methoxime group of MOX. The sites where interactions will be different for MOX compared with IVM are highlighted by a solid red circle. The 25-dimethly-butyl of MOX may also affect interaction with the GluCl (dashed red circle). IVM is a mixture of C25 ethyl (B1a; ∼10%) and C25 methyl (B1b; ∼90%). Against nematodes the B1b component is usually more potent than the B1a component, showing that the change from methyl to ethyl at this position affects potency. Thus it is likely that the 25-dimethyl-butyl group of MOX will also affect the interaction with a GluCl. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
