Graphical abstract
Highlights
► In silico pipeline for the identification of essential and selectively druggable targets. ► Prediction of gene essentiality based on Plasmodium spp. phylogenomics. ► Successful testing of the algorithm based on yeast functional genomics. ► 40 candidate drug targets identified from P. falciparum including known and new ones. ► 23 of these have a positive druggability score, inhibitors inferred against 15.
Keywords: Plasmodium falciparum, Phylogenomics, Gene essentiality, Drug target
Abstract
The need for new antimalarials is persistent due to the emergence of drug resistant parasites. Here we aim to identify new drug targets in Plasmodium falciparum by phylogenomics among the Plasmodium spp. and comparative genomics to Homo sapiens. The proposed target discovery pipeline is largely independent of experimental data and based on the assumption that P. falciparum proteins are likely to be essential if (i) there are no similar proteins in the same proteome and (ii) they are highly conserved across the malaria parasites of mammals. This hypothesis was tested using sequenced Saccharomycetaceae species as a touchstone. Consecutive filters narrowed down the potential target space of P. falciparum to proteins that are likely to be essential, matchless in the human proteome, expressed in the blood stages of the parasite, and amenable to small molecule inhibition. The final set of 40 candidate drug targets was significantly enriched in essential proteins and comprised proven targets (e.g. dihydropteroate synthetase or enzymes of the non-mevalonate pathway), targets currently under investigation (e.g. calcium-dependent protein kinases), and new candidates of potential interest such as phosphomannose isomerase, phosphoenolpyruvate carboxylase, signaling components, and transporters. The targets were prioritized based on druggability indices and on the availability of in vitro assays. Potential inhibitors were inferred from similarity to known targets of other disease systems. The identified candidates from P. falciparum provide insight into biochemical peculiarities and vulnerable points of the malaria parasite and might serve as starting points for rational drug discovery.
1. Introduction
Drug discovery programs launched by the Medicines for Malaria Venture and other product-development partnerships have culminated in the development of promising new antimalarial compounds such as the synthetic peroxide OZ439 (Charman et al., 2011) and the spiroindolone NITD 609 (Rottmann et al., 2010), which are currently undergoing clinical trials. In spite of these recent successes, it is pivotal to maintain early phase drug discovery to prevent the antimalarial drug development pipeline from draining. Due to the propensity of the parasite to become drug-resistant (Muller and Hyde, 2010; Sa et al., 2011), the need for new antimalarial chemotypes will persist until the human-pathogenic Plasmodium spp. are eventually eradicated.
Rational post-genomic drug discovery is based on the screening of large chemical libraries – either virtually or in high-throughput format – against a given target enzyme of the parasite. A persistent bottleneck for target-based approaches is the identification of a suitable drug target in the first place. This enzyme should be essential for survival of the parasite and sufficiently different from its closest counterpart in the human host to be inhibited selectively. Experimental tools to validate candidate drug targets are limited for the malaria parasites. Gene silencing by RNAi does not seem to be feasible (Baum et al., 2009). Gene replacement with selectable markers is (Triglia et al., 1998), but it is inherently problematic to call a gene essential from failing to knock it out. Inducible degradation of Plasmodium falciparum proteins that have been fused to a FKBP-destabilization domain (Armstrong and Goldberg, 2007) is currently the most conclusive method for antimalarial target validation. However, none of the reverse genetic methods is practicable at the genome-wide scale. Adding up to the challenges with Plasmodium molecular biology is the lack of a phylogenetically close model organism that could serve as a point of reference – as is the case with parasitic nematodes, where essentiality of genes may be estimated based on the RNAi phenotypes (Schindelman et al., 2011) of orthologues in Caenorhabditis elegans.
Several bioinformatic approaches have previously been employed to help identify or prioritize drug targets for Plasmodium parasites. These include techniques based on automated identification of important steps in metabolic pathways (Yeh et al., 2004; Fatumo et al., 2009; Huthmacher et al., 2010; Plata et al., 2010), techniques that combine chemical starting points and protein-based queries (Joubert et al., 2009), as well as the use of the TDRtargets web-resource (http://www.tdrtargets.org) (Magarinos et al., 2012) to prioritize drug targets through the combination of multiple data types relevant to drug development (Crowther et al., 2010).
Here we try to predict antimalarial drug targets in silico, building on previous approaches by other labs for predicting essentiality of proteins based on phylogeny (Doyle et al., 2010; Waterhouse et al., 2010). We define a protein as a candidate antimalarial drug target if it (i) has conserved orthologues in all of the mammalian-pathogenic Plasmodium spp.; (ii) has no other match in P. falciparum; (iii) does not have a good match in the human proteome either; (iv) is expressed in the trophozoite and gametocyte stages; and (v) is predicted to function as an enzyme, receptor, or transporter. The rationale is that conserved genes often fulfill essential functions – or rather that genes fulfilling essential functions are under negative selection. A conserved single copy gene that lacks close matches in the same genome is likely to be indispensable. Starting from the complete predicted proteome of P. falciparum (Gardner et al., 2002), we applied consecutive filters to extract all candidate drug targets that meet the above criteria.
2. Material and methods
2.1. Datasets
The predicted Plasmodium spp. proteomes were downloaded from PlasmoDB (http://www.plasmodb.org/common/downloads) (Aurrecoechea et al., 2009), the Saccharomyces cerevisiae proteome from SGD (Saccharomyces genome database; http://www.downloads.yeastgenome.org/) (Engel et al., 2010), the Homo sapiens proteome from EBI (ftp://www.ftp.ebi.ac.uk/pub/databases/integr8/fasta/proteomes) (Mulder et al., 2008), and all others from UniProt (http://www.uniprot.org/taxonomy) (Magrane and Consortium, 2011). P. falciparum 3D7 cell cycle expression data (Le Roch et al., 2003) were obtained from PlasmoDB, using as a threshold for expression E > 10 and probe signal distribution log P < −0.5 as proposed by the authors (Le Roch et al., 2003). S. cerevisiae deletion phenotype data were from SGD (http://www.downloads.yeastgenome.org/curation/literature/phenotype_data.tab). Proteins were termed essential if the phenotype of the knock-out (mutant type = ‘null’) of the corresponding gene was ‘inviable’. The TDRtargets web resource (http://www.tdrtargets.org) (Magarinos et al., 2012), as well as the BRENDA database (http://www.brenda-enzymes.org) (Scheer et al., 2011) was used to identify proteins with precedence for interaction with small molecule chemical inhibitors. Essentiality of Plasmodium berghei orthologues was found through RMgmDB (Rodent Malaria genetically modified Parasites; http://www.pberghei.eu/) (Janse et al., 2011).
2.2. Programs
InParanoid was obtained from http://www.inparanoid.sbc.su.se and QuickParanoid from http://www.pl.postech.ac.kr/QuickParanoid. BLAST 2.2.17 (Altschul et al., 1990) was downloaded from NCBI (ftp.ncbi.nlm.nih.gov), Needle (from the EMBOSS suite (Rice et al., 2000)) from http://www.emboss.sourceforge.net. All programs were run on the Basel Biocomputing Linux cluster (http://www.bc2.unibas.ch).
2.3. Algorithms
The pipeline for comparative genomics and target prediction was run in a semi-automated way, combining the existing programs (see Section 2.2) with self-made Perl scripts for parsing, re-formatting, and analysis of outputs. Druggability scores were extracted from the TDRtargets website (Magarinos et al., 2012). These druggability predictions are numerical values between 0 and 1, with 1 corresponding to more druggable targets. Scores reflect a combination of factors including degree of similarity to a large library of targets within the ChEMBL database (Hopkins et al., 2011; Gaulton et al., 2012), with empirically determined interactions with drug like compounds, possession of physiochemical features enriched in known drug targets, and the drug-like quality of the inhibitors known to interact with those homologues. These scores are outputs of a combination of multiple discriminators based on empirically-characterized drug targets, but are untested in Plasmodium, other than through concordance of high-scoring proteins with the very few known antimalarial drug targets. Proteins with higher druggability scores are likely to be more amenable to inhibition with drug-like molecules than proteins with low druggability scores, and hence are used here as one aspect of the ranking system, but there is lack of parasite-specific direct data for these algorithms with which to assign a cutoff for designating an acceptable score.
3. Results and discussion
3.1. Using yeast as a touchstone for essentiality prediction
The central hypothesis implemented in the present target discovery pipeline is, that a protein is likely to be essential if there are no other similar proteins in the same proteome but conserved orthologues in all the related species. Before applying it to malaria parasites, we tested the hypothesis on the yeast S. cerevisiae where essentiality of proteins has been tested by systematic knock-out of the respective genes (Winzeler et al., 1999). The current null mutant dataset in the Saccharomyces Genome Database comprises 5477 genes, of which 1109 were found to be essential. To test whether a given S. cerevisiae protein is unique, its best match (excluding itself) in the same proteome was identified with Blastp (Altschul et al., 1990), then and a Needleman-Wunsch global alignment (Needleman and Wunsch, 1970) was performed between the two proteins. The median global identity between a S. cerevisiae protein and its best match was 15.7% (red bar in Fig. 1). 14% of the proteins with a match above the median were essential, compared to 24% of those below the median (Fig. 1). The difference was significant (p < 0.0001, two-sided Chi-square test) and supported the hypothesis that ‘unmatched’ proteins are more likely to be essential (Hannay et al., 2008). Setting the cut-off at ⩽15% global identity 2648 S. cerevisiae proteins passed (black area in Fig. 1), 26% of which were essential (Fig. 2).
Fig. 1.
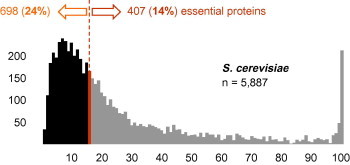
S. cerevisiae non-redundant proteins. Frequency distribution of the similarities for all predicted S. cerevisiae proteins to their next best match in the same proteome. Of the 1109 essential proteins, 698 were below the median identity of 15.7% (red bar) and 407 above (4 proteins had exactly 15.7% identity to their next best match). The black area represents the proteins below the threshold of ⩽15% global identity. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
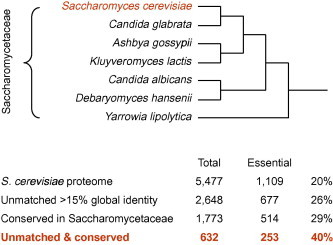
Testing for essentiality with yeasts. The cladogram depicts the phylogeny of the fully sequenced Saccharomycetaceae (Fitzpatrick et al., 2006). Eliminating the S. cerevisiae proteins which possess matches in the same proteome (gray area in Fig. 1), and those which are not conserved across all the analyzed yeasts, enriches for essential proteins.
To determine which of the S. cerevisiae proteins are conserved across the Saccharomycetaceae, a set of related (Fitzpatrick et al., 2006) and fully sequenced yeasts served as the reference: Candida glabrata, Ashbya gossypii, Kluyveromyces lactis, Candida albicans, Debaryomyces hansenii, and Yarrowia lipolytica. The predicted proteomes (Table 1) were fed into InParanoid and QuickParanoid (Ostlund et al., 2010), programs that utilize reciprocal, proteome-wide Blastp searches to build groups of orthologous proteins and rank them. A total of 3546 S. cerevisiae proteins possessed orthologues in all the included yeasts. The highly conserved orthology clusters, i.e. those above median similarity, were significantly enriched for essential proteins (Fig. 2): 29% of the S. cerevisiae proteins that belonged to a conserved cluster were essential (p < 0.0001, two-sided Chi-square test). Finally the intersect of the two sets, unmatched and conserved, contained 632 proteins of which 40% were essential (Fig. 2). The yeast results supporting our working hypothesis, we went on to apply it to malaria parasites.
Table 1.
Proteomes used in this study, their sizes and sources.
| Species | Proteins | Source | Version |
|---|---|---|---|
| P. falciparum | 5410 | PlasmoDB | 8.1 |
| P. vivax | 5396 | PlasmoDB | 8.1 |
| P. berghei | 4861 | PlasmoDB | 8.1 |
| P. chabaudi | 5119 | PlasmoDB | 8.1 |
| P. knowlesi | 5194 | PlasmoDB | 8.1 |
| P. yoelii | 7724 | PlasmoDB | 8.1 |
| H. sapiens | 67,172 | Integr8 | 08/2011 |
| S. cerevisiae | 5887 | SGD | R64-1-1 |
| A. gossypii | 4761 | UniProt | 08/2011 |
| C. albicans | 5727 | UniProt | 08/2011 |
| C. glabrata | 5197 | UniProt | 08/2011 |
| D. hansenii | 6242 | UniProt | 08/2011 |
| K. lactis | 5074 | UniProt | 08/2011 |
| Y. lipolytica | 6414 | UniProt | 08/2011 |
3.2. Essentiality prediction in P. falciparum
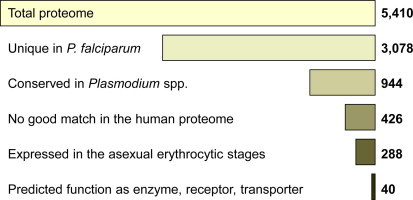
The predicted P. falciparum proteome exhibits a strikingly low degree of redundancy, with the large majority of proteins exhibiting but a low level of similarity to their next best match in the same proteome (Fig. 3). As described above (Section 3.1) for S. cerevisiae, the closest hit to any P. falciparum protein was determined with Blastp. Similarity was measured by global alignment and expressed as percent identical amino acids. The median global identity between a P. falciparum protein and its next best match was only 13% (red bar in Fig. 3), and 3078 of the 5410 proteins passed the threshold of ⩽15% global identity to their next best match (black area in Fig. 3).
Fig. 3.
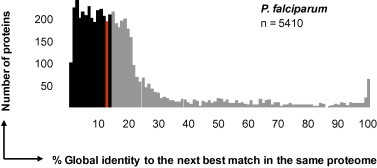
P. falciparum non-redundant proteins. Frequency distribution of the similarities for all predicted P. falciparum proteins to their next best match in the same proteome. The median is shown in red, the black area represents the proteins which, passing the threshold of ⩽15% global identity, are assumed to be enriched for essential ones.
The conserved Plasmodium proteins were identified by comparing P. falciparum to all other available genome sequences of mammalian malaria parasites, namely P. berghei, Plasmodium chabaudi and Plasmodium yoelii (mouse), Plasmodium knowlesi (primate), and Plasmodium vivax (human tertian malaria). The six genomes encode a total of 33,704 predicted proteins (Table 1). The common intersect of proteins from orthology clusters represented in all the six species was 26,377, as determined with InParanoid and QuickParanoid (Ostlund et al., 2010). This amounts to 78% of all proteins, reflecting the close relationship of the malaria parasites. The number of proteins in the common clusters was conserved in all the analyzed Plasmodium spp., and the size of their proteomes correlated with the number of species-specific proteins (Fig. 4). Of the 5410 predicted P. falciparum proteins, 3610 (67%) possessed at least one orthologue in all of the other Plasmodium species (Fig. 4). 630 P. falciparum proteins did not have an orthologue in any other species and 139 had an orthologue in P. vivax and P. knowlesi but not in the malaria parasites of rodents (Fig. 4). The different protein subsets outlined in Fig. 4 for each parasite are available as a Supplementary excel file (supplement1.xls). The intersect of the two subsets, P. falciparum unique (n = 3078) and P. falciparum highly conserved (i.e. above median, n = 1805), contained 944 proteins which we posit – based on the results with S. cerevisiae – to be enriched for essential ones.
Fig. 4.
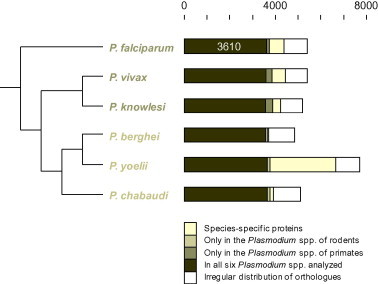
The conserved malaria proteome. The cladogram depicts the phylogeny of the fully sequenced malaria parasites of mammals (Martinsen et al., 2008). The bars divide each predicted proteome into proteins present only in the species itself, proteins with orthologues only in the Plasmodium spp. sharing intermediate hosts, and proteins with orthologues in all the analyzed species (3610 in P. falciparum). The white sections represent proteins with an irregular distribution insofar as the presence of orthologues did not correlate with host specificity (nor with phylogeny).
3.3. Comparative genomics between parasite and host
For each predicted protein of P. falciparum the best match in the human proteome was identified with Blastp, ranking the obtained hits by ascending expectancy (E-values). The similarity between a P. falciparum protein and this closest match from H. sapiens was then quantified with a Needleman-Wunsch global alignment (since the local alignment provided by Blast may be misleading if the similarity between two otherwise unrelated proteins is confined to a smaller common domain). The frequency distribution of global identity to human proteins in the P. falciparum proteome is shown in Fig. 5. The most conserved proteins between parasite and host were ubiquitins, histones, calmodulin, and tubulin. The median global identity between a P. falciparum protein and its best match in H. sapiens was 10% (red bar in Fig. 5). Proteins exhibiting >10% identity to the host (gray area in Fig. 5) were removed from the potential target space, which thereby was trimmed down to 426 proteins (Fig. 6). Some of these still had highly significant Blastp E-values to a human protein, e.g. the P. falciparum kinases listed in Table 2, since a short region of high similarity in two otherwise disparate proteins can return a high local alignment score (in the case of the P. falciparum kinases, this region is the catalytic domain). Nevertheless, selective targeting might be possible against other domains.
Fig. 5.
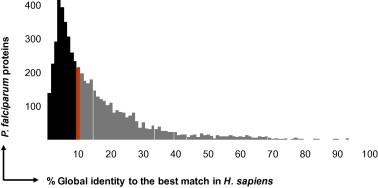
Parasite vs. host. Frequency distribution of percent global identity to H. sapiens in the P. falciparum proteome. The red bar marks the median, the gray areas the P. falciparum proteins that were excluded from the potential target space.
Fig. 6.
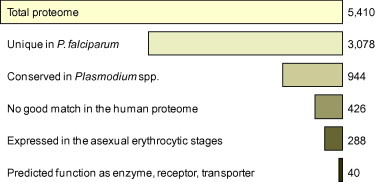
From genome to target. Overview on the in silico pipeline to narrow down the potential target space of P. falciparum. The 40 identified candidates are listed in Table 2.
Table 2.
Forty candidate drug targets from P. falciparum identified as outlined in Fig. 6, their putative function, Blastp E-value to the best hit in the human proteome, and druggability index (D.I.). The categories (italics) are arbitrary and mainly meant to illustrate the functional diversity of the identified targets.
| Accession | Putative function | %Id Hsa | E Hsa | D.I. |
|---|---|---|---|---|
| Metabolism | ||||
| PF08_0095 | Dihydropteroate synthetase (2.5.1.15) | 0.8 | 8.5 | 0.8 |
| MAL8P1.156 | Mannose-6-P isomerase (5.3.1.8) | 6.9 | 1E-8 | 0.6 |
| PF08_0068 | FAD-dependent monooxygenase | 6.0 | 0.07 | 0.5 |
| PF14_0334 | Ornithine aminotransferase (2.6.1.13) | 4.3 | 1E-14 | 0.3 |
| PF13_0234 | PEP carboxykinase (4.1.1.49) | 4.0 | 3.6 | 0.2 |
| PF14_0246 | PEP carboxylase (4.1.1.31) | 3.8 | 4.5 | 0.1 |
| PFI1180w | Patatin-like phospholipase | 6.3 | 7.4 | 0.1 |
| PF10_0221 | HMB-PP synthase (GcpE, 1.17.7.1) | 6.5 | 2.2 | |
| PFA0225w | HMB-PP reductase (1.17.1.2) | 7.3 | 5.3 | |
| MAL7P1.88 | Thioredoxin-like protein | 3.1 | 4.9 | |
| PFI1340w | Fumarate hydratase (Fumarase, 4.2.1.2) | 10.0 | 2.7 | |
| PF08_0132 | Glutamate dehydrogenase C (1.4.1.2) | 8.1 | 7.2 | |
| PFD0285c | Lysine decarboxylase (4.1.1.18) | 5.6 | 7.4 | |
| PFL0620c | Glycerol-3-P acyltransferase (2.3.1.15) | 9.8 | 1.0 | |
| PF14_0250 | Lipase | 3.3 | 8.1 | |
| Nucleic acids | ||||
| PF14_0273 | RNA methyltransferase | 9.8 | 7E-30 | 0.7 |
| PF13_0080 | Telomerase reverse transcriptase (2.7.7.49) | 0.3 | 0.04 | 0.5 |
| PF13_0176 | Apur./apyr. endonuclease (4.2.99.18) | 2.6 | 2.6 | 0.2 |
| MAL13P1.311 | Exonuclease | 5.9 | 7E-4 | 0.1 |
| MAL13P1.328 | DNA topoisomerase VI, b subunit | 4.9 | 7.1 | |
| PFE0675c | DNA photolyase (4.1.99.3) | 3.7 | 0.06 | |
| PF13_0048 | NUDIX hydrolase (3.6.1.17) | 8.4 | 8E-35 | |
| PF08_0111 | RNA helicase | 7.8 | 8E-15 | |
| PFC0980c | RNA triphosphatase | 8.5 | 8.6 | |
| Signaling | ||||
| PF11_0239 | Ca2+-dependent protein kinase 6 | 7.8 | 9E-34 | 0.5 |
| PF11_0242 | Ca2+-dependent protein kinase 7 | 9.0 | 6E-41 | 0.5 |
| PF13_0258 | Serine/threonine protein kinase TKL3 | 4.8 | 3E-21 | 0.5 |
| PFA0515w | Phosphatidylinositol-4-P-5-kinase (2.7.1.68) | 7.0 | 1E-24 | 0.5 |
| PF13_0166 | Protein kinase | 8.9 | 7E-4 | 0.3 |
| PF07_0024 | Inositol phosphatase | 7.3 | 1E-31 | 0.3 |
| MAL7P1.18 | Serine/threonine protein kinase | 4.7 | 5E-13 | 0.1 |
| MAL7P1.64 | G protein-coupled receptor | 2.5 | 93 | 0.1 |
| PF14_0143 | Atypical protein kinase, ABC-1 family | 3.8 | 4E-26 | |
| PF14_0614 | Phosphatase | 6.1 | 4E-5 | |
| Protease/chaperone | ||||
| PF14_0382 | Stromal-processing peptidase | 4.4 | 0.001 | 0.5 |
| PF10_0032 | DnaJ protein | 2.4 | 7E-4 | |
| Transporter | ||||
| PF11_0092 | Mechanosensitive ion channel | 5.0 | 11 | 0.1 |
| PF13_0019 | Na+:H+ antiporter | 6.6 | 0.01 | 0.1 |
| PFI0785c | Sugar transporter | 2.8 | 0.09 | 0.1 |
| PFE0785c | Metabolite transporter/glideosome component | 0.7 | 10 | |
3.4. Blood-stage expression of putative targets
The majority of genes in P. falciparum are expressed in a stage-dependent manner (Llinas and DeRisi, 2004), and it is in the asexual and gametocyte stages that we attempt to find targets given their roles in pathology and transmission. We relied on a published microarray dataset (Le Roch et al., 2003) of P. falciparum cultures synchronized by sorbitol treatment to identify the potential targets that are present in the blood stages. Retaining only the products of genes which are expressed in the ring stages, trophozoites, or schizonts, reduced the target space to 288 proteins (Fig. 6). The complete list is available as a Supplementary excel file (supplement2.xls). Over half of these (n = 178) could not be assigned a putative function. Although of potential interest since conserved in the malaria parasites and absent in humans, these proteins were (for the time being) removed from the candidate target set. Other proteins were removed manually because they were not likely to have a function that is susceptible to inhibition, e.g. structural proteins or surface antigens. Focusing on proteins with a clear, though sometimes putative, functional annotation in PlasmoDB as enzyme, receptor or transporter resulted in a final set of 40 P. falciparum candidate drug targets (Fig. 6).
3.5. Assessing essentiality of the 40 identified candidate targets
For four of the predicted essential proteins attempts have been made to disrupt the corresponding gene in P. falciparum (Table 3). In three cases disruption was attempted through deletion by recombination but no gene deletion could be obtained (Santiago et al., 2004; Wang et al., 2004; Abdi et al., 2010). For the fourth gene ispH (HMBPP reductase; PFA0225w), introduction of a hammerhead ribozyme targeting the transcript was lethal (Vinayak and Sharma, 2007). Thus, each of these four genes experimentally examined appeared to be essential. Similarly, a small number of these proteins’ orthologues in P. berghei or Toxoplasma gondii have been the subject of directed gene knock-out or mutagenesis experiments aimed at assaying their essentiality. The orthologues of the kinases PF13_0166 and CDPK7 (PF11_0242) have been suggested to be essential in P. berghei (Tewari et al., 2010). In T. gondii, the orthologue of the apurinic/apyrimidinic endonuclease (PF13_0176) has been shown to essential by conditional knockout (Onyango et al., 2011) and GAP40 (PFE0785c) is part of the essential glideosome complex, though the protein itself has not been verified as essential (Frenal et al., 2010). Only one gene was found to be non-essential. The P. berghei CDPK6 (PF11_0239) orthologue has been successfully deleted by double crossover (Coppi et al., 2007). These experimental assays for essentiality thus indicate eight of the predicted proteins to be essential, and only one to be non-essential, supporting our method to enrich for essentiality.
Table 3.
Identified targets, evidence for essentiality in apicomplexan parasites (Pfa, P. falciparum; Pbe, P. berghei; Tgo, T. gondii), known and inferred inhibitors, and published in vitro assays.
3.6. Prediction of druggability
In order to recognize the important role of chemical accessibility in ranking of putative drug targets we looked for druggability information on the predicted essential targets. Druggability describes the properties of a protein that make them able to interact with a drug-like molecule. Some proteins have physiochemical and structural properties that make them unlikely to ever bind a compound with the necessary characteristic to be a drug, and whole genome analyses have estimated that only 10% of a genome may represent druggable proteins (Hopkins and Groom, 2002). As part of the TDRtargets project a druggability score was assigned to enzymes from the P. falciparum proteome (Aguero et al., 2008; Magarinos et al., 2012). This score predicts the suitability of the protein for binding a drug like molecule, and is an aggregate of several factors including physiochemical and structural features of the protein and sequence similarity to validated druggable proteins from other organisms (Al-Lazikani et al., 2007; Gaulton et al., 2012). Of the 40 targets predicted as essential, 23 have positive druggability scores (Table 2). Those with the highest scores are predicted to have more druggable structures and might therefore be prioritized over potential targets with low or zero druggability scores. The higher druggability scores are more likely to be indicative of promising drug target-like proteins, but any positive score indicates structural or physiochemical similarity to proteins with some precedence for interaction with a drug-like molecule.
To search for proteins with specific compounds as starting points for inhibitor development we also searched for proteins that had been identified as the targets of inhibitors, or that had orthologues with identified inhibitors. Searches were conducted of the TDRTargets database, the ChEMBL database and the BRENDA database to identify such targets. Of the 40 predicted essential P. falciparum genes, 2 have been directly validated as the targets of inhibitors. One of these, dihydropteroate synthetase (DHPS; PF08_0095) is one of the very few validated targets of clinically used antimalarials, consistent with the value of our approach for identifying essential targets. An additional 13 proteins have orthologues with identified small molecule inhibitors. Identification of these inhibitors not only provides useful starting points for identifying small molecules with which to interrogate the Plasmodium targets, but also indicates that these are likely to be potentially druggable targets. Compounds were curated to exclude facile inhibitors such as natural amino acids, ATP and NaCl, however no systematic attempt was made to rate the drug-like quality of the predicted inhibitors. For the kinases in the list a slightly modified approach was taken, as many kinase inhibitors are well known to be cross reactive against multiple kinases. An empirical cut-off of 30% local sequence identity in the kinase domains was therefore used to identify homologues for potential inhibitors of from the TDR/ChEMBL data (Table 3).
3.7. Experimental amenability of the predicted targets
Tractability of a target for developing an assay in which inhibition is a major factor in prioritizing promising drug targets in infectious diseases because the smaller research communities, compared to those working on human proteins, means fewer protein reagents are available. This is exacerbated in malaria research because the P. falciparum genome is extremely A + T rich, and many proteins have long low-complexity insertions, making recombinant protein production challenging. High throughput attempts to generate recombinant Plasmodium enzymes have relatively high failure rates (Abdi et al., 2010), so the presence of an existing assay is a particularly relevant criteria for target prioritization. We therefore prioritized proteins where researchers had already developed a direct in vitro assay for the P. falciparum enzyme. Eleven of the 40 predicted essential enzymes have had in vitro assays described in P. falciparum, although in some cases these may not be readily amenable to high throughput inhibitor screening.
3.8. Candidate antimalarial drug targets
The approach presented here, although starting from the complete proteome, is by no means all-embracing in the sense that it would discover every potential drug target. Many essential proteins and known drug targets were missed, such as P. falciparum enzymes which possess human orthologues but exhibit exploitable differences nevertheless. These could be differences in sequence, as is the case with glyceraldehyde-3-phosphate dehydrogenase (Satchell et al., 2005) or in regulation, as demonstrated for dihydrofolate reductase (Zhang and Rathod, 2002). Other proven targets were eliminated because they possess several paralogues, e.g. the falcipains (Teixeira et al., 2011). Using rigorous filters, we tried to maximize the specificity of target prediction at the cost of sensitivity, taking into account a large number of false negatives. Our aim was not to identify all potential antimalarial drug targets but rather to come up with a sizeable list of promising targets that (i) may serve as starting points for rational drug discovery and (ii) provide new insights into peculiarities and vulnerable points of the malaria parasites. The applied selection criteria were rigorous (Fig. 6), so the list of potential targets could be expanded by using less stringent filters. The present approach identified 40 candidate drug targets from P. falciparum which are shown in Table 2. We are confident that this set contains promising leads because it includes known targets such as dihydropteroate synthetase (PF08_0095), the target of sulfonamides (dihydrofolate synthetase is not included because it is 20% identical to human mitochondrial tetrahydrofolylpolyglutamate synthase, EC 6.3.2.17), and enzymes of the non-mevalonate pathway for isoprenoid synthesis. Another potential target in the apicoplast could be stromal processing peptidase (PF14_0382), which has already been characterized in P. falciparum (van Dooren et al., 2002). Investigational targets identified include glutamate dehydrogenase C (PF08_0132) (Aparicio et al., 2010; Storm et al., 2011) and Ca2+-dependent protein kinases (PF11_0239, PF11_0242) (Kato et al., 2008; Ojo et al., 2010; Lim et al., 2012). In addition, there are enzymes which are being targeted in other systems, e.g. apurinic/apyrimidinic endonuclease (PF13_0176) (Tell et al., 2010) or telomerase reverse transcriptase (PF13_0080) (Chen et al., 2009) by antitumor agents, or phosphomannose isomerase (MAL8P1.156) by antifungals. Phosphomannose is a key enzyme in yeast cell wall synthesis (Mastrolorenzo et al., 2000; Wills et al., 2001); to our knowledge, the P. falciparum orthologue has so far not been studied. A surprising find in the list of candidate targets was phosphoenolpyruvate carboxylase (PF14_0246), the enzyme responsible for carbon fixation in C4 plants. The P. falciparum orthologue was characterized and proposed to fix atmospheric carbon as well (McDaniel and Siu, 1972). If this process is essential, PEP carboxylase could be an attractive target since inhibitors have been identified as C4 plant herbicides (Madhusudana et al., 1980; Jenkins, 1989; Pairoba et al., 1996). Further candidate targets of interest could be the identified signaling enzymes and nutrient transporters (Table 2).
4. Conclusion
Translating a pathogen’s genome sequence into new drugs is a key challenge to post-genomic biology. Several strategies for the prediction of drug targets that are essential and unique to the parasite have been proposed (Payne et al., 2001; Joyce and Palsson, 2008; Holman et al., 2009; Crowther et al., 2010; Deng et al., 2010; Doyle et al., 2010; Magarinos et al., 2012). Here we implement phylogenomic comparison within a clade of related parasites to infer on protein essentiality, rather than referring to a model organism which may be understood in detail but phylogenetically distant to the parasites. In addition, we estimate redundancy across the parasite’s proteome based on global alignments to quantify protein similarity. Thus the proposed target identification pipeline does not rely on experimental data to predict essentiality, and therefore is generally applicable. The identified candidate targets from P. falciparum are, by definition, present in all the Plasmodium spp. analyzed. Conservation of targets between P. falciparum and P. vivax is important since eventual new drugs need to be active against both parasites. Conservation of the candidate targets between human- and rodent-pathogenic Plasmodium spp. is desirable as well, since mouse models are indispensable in the drug development process. The presented target discovery pipeline is corroborated by the presence of known antimalarial targets in the output list (Table 2). We hope that the newly identified candidate targets will support the development of novel antimalarials.
Acknowledgments
We wish to thank the Basel Computational Biology Center for runtime on their Linux cluster. SAR is funded by an Australian Research Council Future Fellowship (FT0990350). BJW was supported by a University of Melbourne Research scholarship.
Contributor Information
Philipp Ludin, Email: philipp.ludin@unibas.ch.
Ben Woodcroft, Email: b.woodcroft@uq.edu.au.
Stuart A. Ralph, Email: saralph@unimelb.edu.au.
Pascal Mäser, Email: pascal.maeser@unibas.ch.
Appendix A. Supplementary data
References
- Abdi A., Eschenlauer S., Reininger L., Doerig C. SAM domain-dependent activity of PfTKL3, an essential tyrosine kinase-like kinase of the human malaria parasite Plasmodium falciparum. Cell. Mol. Life Sci. 2010;67:3355–3369. doi: 10.1007/s00018-010-0434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari S., Manthena P.V., Kota K.K., Karmahapatra S.K., Roy G., Saxena R., Uren A., Roy R. A comparative study of recombinant mouse and human apurinic/apyrimidinic endonuclease. Mol. Cell. Biochem. 2012;362:195–201. doi: 10.1007/s11010-011-1142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguero F., Al-Lazikani B., Aslett M., Berriman M., Buckner F.S., Campbell R.K., Carmona S., Carruthers I.M., Chan A.W., Chen F., Crowther G.J., Doyle M.A., Hertz-Fowler C., Hopkins A.L., McAllister G., Nwaka S., Overington J.P., Pain A., Paolini G.V., Pieper U., Ralph S.A., Riechers A., Roos D.S., Sali A., Shanmugam D., Suzuki T., Van Voorhis W.C., Verlinde C.L. Genomic-scale prioritization of drug targets: the TDR Targets database. Nat. Rev. Drug Discov. 2008;7:900–907. doi: 10.1038/nrd2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Lazikani, B., Gaulton, A., Paolini, G., Lanfear, J., Overington, J., Hopkins, A.L. (2007). The molecular basis of predicting druggability. In: Bioinformatics – From Genomes to Therapies. In: The Holy Grail: Molecular Function, vol. 3, Wiley, T.L. Weinheim, pp. 1315–1334.
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Aparicio I.M., Marin-Menendez A., Bell A., Engel P.C. Susceptibility of Plasmodium falciparum to glutamate dehydrogenase inhibitors–a possible new antimalarial target. Mol. Biochem. Parasitol. 2010;172:152–155. doi: 10.1016/j.molbiopara.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Armstrong C.M., Goldberg D.E. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nat. Methods. 2007;4:1007–1009. doi: 10.1038/nmeth1132. [DOI] [PubMed] [Google Scholar]
- Aurrecoechea C., Brestelli J., Brunk B.P., Dommer J., Fischer S., Gajria B., Gao X., Gingle A., Grant G., Harb O.S., Heiges M., Innamorato F., Iodice J., Kissinger J.C., Kraemer E., Li W., Miller J.A., Nayak V., Pennington C., Pinney D.F., Roos D.S., Ross C., Stoeckert C.J., Jr., Treatman C., Wang H. PlasmoDB: a functional genomic database for malaria parasites. Nucleic Acids Res. 2009;37:D539–543. doi: 10.1093/nar/gkn814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bapat A., Glass L.S., Luo M., Fishel M.L., Long E.C., Georgiadis M.M., Kelley M.R. Novel small-molecule inhibitor of apurinic/apyrimidinic endonuclease 1 blocks proliferation and reduces viability of glioblastoma cells. J. Pharmacol. Exp. Ther. 2010;334:988–998. doi: 10.1124/jpet.110.169128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum J., Papenfuss A.T., Mair G.R., Janse C.J., Vlachou D., Waters A.P., Cowman A.F., Crabb B.S., de Koning-Ward T.F. Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res. 2009;37:3788–3798. doi: 10.1093/nar/gkp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckmans S., Van Driessche E. Pig heart fumarase contains two distinct substrate-binding sites differing in affinity. J. Biol. Chem. 1998;273:31661–31669. doi: 10.1074/jbc.273.48.31661. [DOI] [PubMed] [Google Scholar]
- Branson K.M., Mertens H.D., Swarbrick J.D., Fletcher J.I., Kedzierski L., Gayler K.R., Gooley P.R., Smith B.J. Discovery of inhibitors of lupin diadenosine 5′,5‴-P(1), P(4)-tetraphosphate hydrolase by virtual screening. Biochemistry. 2009;48:7614–7620. doi: 10.1021/bi900813x. [DOI] [PubMed] [Google Scholar]
- Bulusu V., Jayaraman V., Balaram H. Metabolic fate of fumarate, a side product of the purine salvage pathway in the intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 2011;286:9236–9245. doi: 10.1074/jbc.M110.173328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charman S.A., Arbe-Barnes S., Bathurst I.C., Brun R., Campbell M., Charman W.N., Chiu F.C., Chollet J., Craft J.C., Creek D.J., Dong Y., Matile H., Maurer M., Morizzi J., Nguyen T., Papastogiannidis P., Scheurer C., Shackleford D.M., Sriraghavan K., Stingelin L., Tang Y., Urwyler H., Wang X., White K.L., Wittlin S., Zhou L., Vennerstrom J.L. Synthetic ozonide drug candidate OZ439 offers new hope for a single-dose cure of uncomplicated malaria. Proc. Natl. Acad. Sci. USA. 2011;108:4400–4405. doi: 10.1073/pnas.1015762108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Li Y., Tollefsbol T.O. Strategies targeting telomerase inhibition. Mol. Biotechnol. 2009;41:194–199. doi: 10.1007/s12033-008-9117-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman R.A. Hepatic sn-glycerol-3-phosphate acyltransferases: effect of monoacylglycerol analogs on mitochondrial and microsomal activities. Biochim. Biophys. Acta. 1988;963:367–374. doi: 10.1016/0005-2760(88)90303-7. [DOI] [PubMed] [Google Scholar]
- Coppi A., Tewari R., Bishop J.R., Bennett B.L., Lawrence R., Esko J.D., Billker O., Sinnis P. Heparan sulfate proteoglycans provide a signal to Plasmodium sporozoites to stop migrating and productively invade host cells. Cell Host Microbe. 2007;2:316–327. doi: 10.1016/j.chom.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther G.J., Shanmugam D., Carmona S.J., Doyle M.A., Hertz-Fowler C., Berriman M., Nwaka S., Ralph S.A., Roos D.S., Van Voorhis W.C., Aguero F. Identification of attractive drug targets in neglected-disease pathogens using an in silico approach. PLoS Negl. Trop. Dis. 2010;4:e804. doi: 10.1371/journal.pntd.0000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Deng L., Su S., Zhang M., Lin X., Wei L., Minai A.A., Hassett D.J., Lu L.J. Investigating the predictability of essential genes across distantly related organisms using an integrative approach. Nucleic Acids Res. 2010;39:795–807. doi: 10.1093/nar/gkq784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M.A., Gasser R.B., Woodcroft B.J., Hall R.S., Ralph S.A. Drug target prediction and prioritization: using orthology to predict essentiality in parasite genomes. BMC Genomics. 2010;11:222. doi: 10.1186/1471-2164-11-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel S.R., Balakrishnan R., Binkley G., Christie K.R., Costanzo M.C., Dwight S.S., Fisk D.G., Hirschman J.E., Hitz B.C., Hong E.L., Krieger C.J., Livstone M.S., Miyasato S.R., Nash R., Oughtred R., Park J., Skrzypek M.S., Weng S., Wong E.D., Dolinski K., Botstein D., Cherry J.M. Saccharomyces Genome Database provides mutant phenotype data. Nucleic Acids Res. 2010;38:D433–436. doi: 10.1093/nar/gkp917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatumo S., Plaimas K., Mallm J.P., Schramm G., Adebiyi E., Oswald M., Eils R., Konig R. Estimating novel potential drug targets of Plasmodium falciparum by analysing the metabolic network of knock-out strains in silico. Infect. Genet. Evol. 2009;9:351–358. doi: 10.1016/j.meegid.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D.A., Logue M.E., Stajich J.E., Butler G. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol. Biol. 2006;6:99. doi: 10.1186/1471-2148-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D.H. Initial kinetic and mechanistic characterization of Escherichia coli fumarase A. Arch. Biochem. Biophys. 1994;311:509–516. doi: 10.1006/abbi.1994.1269. [DOI] [PubMed] [Google Scholar]
- Foret J., de Courcy B., Gresh N., Piquemal J.P., Salmon L. Synthesis and evaluation of non-hydrolyzable d-mannose 6-phosphate surrogates reveal 6-deoxy-6-dicarboxymethyl-d-mannose as a new strong inhibitor of phosphomannose isomerases. Bioorg. Med. Chem. 2009;17:7100–7107. doi: 10.1016/j.bmc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Frenal K., Polonais V., Marq J.B., Stratmann R., Limenitakis J., Soldati-Favre D. Functional dissection of the apicomplexan glideosome molecular architecture. Cell Host Microbe. 2010;8:343–357. doi: 10.1016/j.chom.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Gafan C., Wilson J., Berger L.C., Berger B.J. Characterization of the ornithine aminotransferase from Plasmodium falciparum. Mol. Biochem. Parasitol. 2001;118:1–10. doi: 10.1016/s0166-6851(01)00357-7. [DOI] [PubMed] [Google Scholar]
- Gardner M.J., Hall N., Fung E., White O., Berriman M., Hyman R.W., Carlton J.M., Pain A., Nelson K.E., Bowman S., Paulsen I.T., James K., Eisen J.A., Rutherford K., Salzberg S.L., Craig A., Kyes S., Chan M.S., Nene V., Shallom S.J., Suh B., Peterson J., Angiuoli S., Pertea M., Allen J., Selengut J., Haft D., Mather M.W., Vaidya A.B., Martin D.M., Fairlamb A.H., Fraunholz M.J., Roos D.S., Ralph S.A., McFadden G.I., Cummings L.M., Subramanian G.M., Mungall C., Venter J.C., Carucci D.J., Hoffman S.L., Newbold C., Davis R.W., Fraser C.M., Barrell B. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaulton A., Bellis L.J., Bento A.P., Chambers J., Davies M., Hersey A., Light Y., McGlinchey S., Michalovich D., Al-Lazikani B., Overington J.P. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res. 2012;40:D1100–1107. doi: 10.1093/nar/gkr777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A., Starzynska E., McLennan A.G., Baraniak J., Stec W.J. Adenosine-5′-O-phosphorylated and adenosine-5′-O-phosphorothioylated polyols as strong inhibitors of (symmetrical) and (asymmetrical) dinucleoside tetraphosphatases. Biochem. J. 2003;373:635–640. doi: 10.1042/BJ20030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guranowski A., Starzynska E., Pietrowska-Borek M., Rejman D., Blackburn G.M. Novel diadenosine polyphosphate analogs with oxymethylene bridges replacing oxygen in the polyphosphate chain: potential substrates and/or inhibitors of Ap4A hydrolases. FEBS J. 2009;276:1546–1553. doi: 10.1111/j.1742-4658.2009.06882.x. [DOI] [PubMed] [Google Scholar]
- Haltiwanger B.M., Karpinich N.O., Taraschi T.F. Characterization of class II apurinic/apyrimidinic endonuclease activities in the human malaria parasite, Plasmodium falciparum. Biochem. J. 2000;345(Pt 1):85–89. [PMC free article] [PubMed] [Google Scholar]
- Hannay K., Marcotte E.M., Vogel C. Buffering by gene duplicates: an analysis of molecular correlates and evolutionary conservation. BMC Genomics. 2008;9:609. doi: 10.1186/1471-2164-9-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward R.E. Plasmodium falciparum phosphoenolpyruvate carboxykinase is developmentally regulated in gametocytes. Mol. Biochem. Parasitol. 2000;107:227–240. doi: 10.1016/s0166-6851(00)00191-2. [DOI] [PubMed] [Google Scholar]
- Holman A.G., Davis P.J., Foster J.M., Carlow C.K., Kumar S. Computational prediction of essential genes in an unculturable endosymbiotic bacterium, Wolbachia of Brugia malayi. BMC Microbiol. 2009;9:243. doi: 10.1186/1471-2180-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A.L., Groom C.R. The druggable genome. Nat. Rev. Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Hopkins A.L., Bickerton G.R., Carruthers I.M., Boyer S.K., Rubin H., Overington J.P. Rapid analysis of pharmacology for infectious diseases. Curr. Top. Med. Chem. 2011;11:1292–1300. doi: 10.2174/156802611795429130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huthmacher C., Hoppe A., Bulik S., Holzhutter H.G. Antimalarial drug targets in Plasmodium falciparum predicted by stage-specific metabolic network analysis. BMC Syst. Biol. 2010;4:120. doi: 10.1186/1752-0509-4-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse C.J., Kroeze H., van Wigcheren A., Mededovic S., Fonager J., Franke-Fayard B., Waters A.P., Khan S.M. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 2011;27:31–39. doi: 10.1016/j.pt.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Jenkins C.L. Effects of the phosphoenolpyruvate carboxylase inhibitor 3,3-dichloro-2-(dihydroxyphosphinoylmethyl)propenoate on photosynthesis: C(4) selectivity and studies on C(4) photosynthesis. Plant Physiol. 1989;89:1231–1237. doi: 10.1104/pp.89.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joubert F., Harrison C.M., Koegelenberg R.J., Odendaal C.J., de Beer T.A. Discovery: an interactive resource for the rational selection and comparison of putative drug target proteins in malaria. Malar. J. 2009;8:178. doi: 10.1186/1475-2875-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce A.R., Palsson B.O. Predicting gene essentiality using genome-scale in silico models. Methods Mol. Biol. 2008;416:433–457. doi: 10.1007/978-1-59745-321-9_30. [DOI] [PubMed] [Google Scholar]
- Kato N., Sakata T., Breton G., Le Roch K.G., Nagle A., Andersen C., Bursulaya B., Henson K., Johnson J., Kumar K.A., Marr F., Mason D., McNamara C., Plouffe D., Ramachandran V., Spooner M., Tuntland T., Zhou Y., Peters E.C., Chatterjee A., Schultz P.G., Ward G.E., Gray N., Harper J., Winzeler E.A. Gene expression signatures and small-molecule compounds link a protein kinase to Plasmodium falciparum motility. Nat. Chem. Biol. 2008;4:347–356. doi: 10.1038/nchembio.87. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Takematsu H., Yamaji T., Hiramoto S., Kozutsumi Y. Disturbance of sphingolipid biosynthesis abrogates the signaling of Mss4, phosphatidylinositol-4-phosphate 5-kinase, in yeast. J. Biol. Chem. 2005;280:18087–18094. doi: 10.1074/jbc.M414138200. [DOI] [PubMed] [Google Scholar]
- Le Roch K.G., Zhou Y., Blair P.L., Grainger M., Moch J.K., Haynes J.D., De La Vega P., Holder A.A., Batalov S., Carucci D.J., Winzeler E.A. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–1508. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- Leber W., Skippen A., Fivelman Q.L., Bowyer P.W., Cockcroft S., Baker D.A. A unique phosphatidylinositol 4-phosphate 5-kinase is activated by ADP-ribosylation factor in Plasmodium falciparum. Int. J. Parasitol. 2009;39:645–653. doi: 10.1016/j.ijpara.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Levillain O., Diaz J.J., Reymond I., Soulet D. Ornithine metabolism along the female mouse nephron: localization of ornithine decarboxylase and ornithine aminotransferase. Pflugers Arch. 2000;440:761–769. doi: 10.1007/s004240000340. [DOI] [PubMed] [Google Scholar]
- Li M., Allen A., Smith T.J. High throughput screening reveals several new classes of glutamate dehydrogenase inhibitors. Biochemistry. 2007;46:15089–15102. doi: 10.1021/bi7018783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Smith C.J., Walker M.T., Smith T.J. Novel inhibitors complexed with glutamate dehydrogenase: allosteric regulation by control of protein dynamics. J. Biol. Chem. 2009;284:22988–23000. doi: 10.1074/jbc.M109.020222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.C., Cooke B.M., Doerig C., Saeij J.P. Toxoplasma and Plasmodium protein kinases: roles in invasion and host cell remodelling. Int. J. Parasitol. 2012;42:21–32. doi: 10.1016/j.ijpara.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas M., DeRisi J.L. Pernicious plans revealed: Plasmodium falciparum genome wide expression analysis. Curr. Opin. Microbiol. 2004;7:382–387. doi: 10.1016/j.mib.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Madhusudana Rao.I., Swamy P.M., Das V.S.R. Herbricide (sic) inhibited phosphoenolpyruvate carboxylase in leaves of six nonsucculent scrub species. Z. Pflanzenphysiol. 1980;99:69–74. [Google Scholar]
- Magarinos M.P., Carmona S.J., Crowther G.J., Ralph S.A., Roos D.S., Shanmugam D., Van Voorhis W.C., Aguero F. TDR Targets: a chemogenomics resource for neglected diseases. Nucleic Acids Res. 2012;40:D1118–1127. doi: 10.1093/nar/gkr1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrane, M., Consortium, U., 2011. UniProt Knowledgebase: A Hub of Integrated Protein Data. Database, Oxford, bar009. [DOI] [PMC free article] [PubMed]
- Martinsen E.S., Perkins S.L., Schall J.J. A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol. Phylogenet. Evol. 2008;47:261–273. doi: 10.1016/j.ympev.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Mastrolorenzo A., Scozzafava A., Supuran C.T. Antifungal activity of Ag(I) and Zn(II) complexes of aminobenzolamide (5-sulfanilylamido-1,3,4-thiadiazole-2-sulfonamide) derivatives. J. Enzym. Inhib. 2000;15:517–531. doi: 10.3109/14756360009040707. [DOI] [PubMed] [Google Scholar]
- McDaniel H.G., Siu P.M. Purification and characterization of phosphoenolpyruvate carboxylase from Plasmodium berghei. J. Bacteriol. 1972;109:385–390. doi: 10.1128/jb.109.1.385-390.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder N.J., Kersey P., Pruess M., Apweiler R. In silico characterization of proteins: UniProt, InterPro and Integr8. Mol. Biotechnol. 2008;38:165–177. doi: 10.1007/s12033-007-9003-x. [DOI] [PubMed] [Google Scholar]
- Muller I.B., Hyde J.E. Antimalarial drugs: modes of action and mechanisms of parasite resistance. Future Microbiol. 2010;5:1857–1873. doi: 10.2217/fmb.10.136. [DOI] [PubMed] [Google Scholar]
- Needleman S.B., Wunsch C.D. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- Ojo K.K., Larson E.T., Keyloun K.R., Castaneda L.J., Derocher A.E., Inampudi K.K., Kim J.E., Arakaki T.L., Murphy R.C., Zhang L., Napuli A.J., Maly D.J., Verlinde C.L., Buckner F.S., Parsons M., Hol W.G., Merritt E.A., Van Voorhis W.C. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat. Struct. Mol. Biol. 2010;17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onyango D.O., Naguleswaran A., Delaplane S., Reed A., Kelley M.R., Georgiadis M.M., Sullivan W.J., Jr. Base excision repair apurinic/apyrimidinic endonucleases in apicomplexan parasite Toxoplasma gondii. DNA Repair (Amst.) 2011;10:466–475. doi: 10.1016/j.dnarep.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund G., Schmitt T., Forslund K., Kostler T., Messina D.N., Roopra S., Frings O., Sonnhammer E.L. InParanoid 7: new algorithms and tools for eukaryotic orthology analysis. Nucleic Acids Res. 2010;38:D196–203. doi: 10.1093/nar/gkp931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pairoba C.F., Colombo S.L., Andreo C.S. Flavonoids as inhibitors of NADP-malic enzyme and PEP carboxylase from C4 plants. Biosci. Biotechnol. Biochem. 1996;60:779–783. doi: 10.1271/bbb.60.779. [DOI] [PubMed] [Google Scholar]
- Payne D.J., Holmes D.J., Rosenberg M. Delivering novel targets and antibiotics from genomics. Curr. Opin. Investig. Drugs. 2001;2:1028–1034. [PubMed] [Google Scholar]
- Plata G., Hsiao T.L., Olszewski K.L., Llinas M., Vitkup D. Reconstruction and flux-balance analysis of the Plasmodium falciparum metabolic network. Mol. Syst. Biol. 2010;6:408. doi: 10.1038/msb.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice P., Longden I., Bleasby A. EMBOSS: the European molecular biology open software suite. Trends Genet. 2000;16:276–277. doi: 10.1016/s0168-9525(00)02024-2. [DOI] [PubMed] [Google Scholar]
- Riguet E., Desire J., Boden O., Ludwig V., Gobel M., Bailly C., Decout J.L. Neamine dimers targeting the HIV-1 TAR RNA. Bioorg. Med. Chem. Lett. 2005;15:4651–4655. doi: 10.1016/j.bmcl.2005.07.082. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Acosta A., de Dominguez N., Aguilar I., Giron M.E. Detection of glutamate dehydrogenase enzyme activity in Plasmodium falciparum infection. Indian J. Med. Res. 1999;109:152–156. [PubMed] [Google Scholar]
- Rohrich R.C., Englert N., Troschke K., Reichenberg A., Hintz M., Seeber F., Balconi E., Aliverti A., Zanetti G., Kohler U., Pfeiffer M., Beck E., Jomaa H., Wiesner J. Reconstitution of an apicoplast-localised electron transfer pathway involved in the isoprenoid biosynthesis of Plasmodium falciparum. FEBS Lett. 2005;579:6433–6438. doi: 10.1016/j.febslet.2005.10.037. [DOI] [PubMed] [Google Scholar]
- Rottmann M., McNamara C., Yeung B.K., Lee M.C., Zou B., Russell B., Seitz P., Plouffe D.M., Dharia N.V., Tan J., Cohen S.B., Spencer K.R., Gonzalez-Paez G.E., Lakshminarayana S.B., Goh A., Suwanarusk R., Jegla T., Schmitt E.K., Beck H.P., Brun R., Nosten F., Renia L., Dartois V., Keller T.H., Fidock D.A., Winzeler E.A., Diagana T.T. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329:1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sa J.M., Chong J.L., Wellems T.E. Malaria drug resistance: new observations and developments. Essays Biochem. 2011;51:137–160. doi: 10.1042/bse0510137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvati L., Mattu M., Tiberi F., Polticelli F., Ascenzi P. Inhibition of Saccharomyces cerevisiae phosphomannose isomerase by the NO-donor S-nitroso-acetyl-penicillamine. J. Enzym. Inhib. 2001;16:287–292. doi: 10.1080/14756360109162377. [DOI] [PubMed] [Google Scholar]
- Santiago T.C., Zufferey R., Mehra R.S., Coleman R.A., Mamoun C.B. The Plasmodium falciparum PfGatp is an endoplasmic reticulum membrane protein important for the initial step of malarial glycerolipid synthesis. J. Biol. Chem. 2004;279:9222–9232. doi: 10.1074/jbc.M310502200. [DOI] [PubMed] [Google Scholar]
- Satchell J.F., Malby R.L., Luo C.S., Adisa A., Alpyurek A.E., Klonis N., Smith B.J., Tilley L., Colman P.M. Structure of glyceraldehyde-3-phosphate dehydrogenase from Plasmodium falciparum. Acta Crystallogr. D: Biol. Crystallogr. 2005;61:1213–1221. doi: 10.1107/S0907444905018317. [DOI] [PubMed] [Google Scholar]
- Scheer M., Grote A., Chang A., Schomburg I., Munaretto C., Rother M., Sohngen C., Stelzer M., Thiele J., Schomburg D. BRENDA, the enzyme information system in 2011. Nucleic Acids Res. 2011;39:D670–676. doi: 10.1093/nar/gkq1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelman G., Fernandes J.S., Bastiani C.A., Yook K., Sternberg P.W. Worm Phenotype Ontology: integrating phenotype data within and beyond the C. elegans community. BMC Bioinform. 2011;12:32. doi: 10.1186/1471-2105-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwilaijareon N., Petmitr S., Mutirangura A., Ponglikitmongkol M., Wilairat P. Stage specificity of Plasmodium falciparum telomerase and its inhibition by berberine. Parasitol. Int. 2002;51:99–103. doi: 10.1016/s1383-5769(01)00092-7. [DOI] [PubMed] [Google Scholar]
- Storm J., Perner J., Aparicio I., Patzewitz E.M., Olszewski K., Llinas M., Engel P.C., Muller S. Plasmodium falciparum glutamate dehydrogenase a is dispensable and not a drug target during erythrocytic development. Malar. J. 2011;10:193. doi: 10.1186/1475-2875-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranska J., Kopecny D., Tylichova M., Snegaroff J., Sebela M. Ornithine delta-aminotransferase: an enzyme implicated in salt tolerance in higher plants. Plant Signal. Behav. 2008;3:929–935. doi: 10.4161/psb.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker H.J. Purification and properties of rat liver ornithine delta-transaminase. J. Biol. Chem. 1965;240:1225–1230. [PubMed] [Google Scholar]
- Takatsuka Y., Onoda M., Sugiyama T., Muramoto K., Tomita T., Kamio Y. Novel characteristics of Selenomonas ruminantium lysine decarboxylase capable of decarboxylating both l-lysine and l-ornithine. Biosci. Biotechnol. Biochem. 1999;63:1063–1069. doi: 10.1271/bbb.63.1063. [DOI] [PubMed] [Google Scholar]
- Teixeira C., Gomes J.R., Gomes P. Falcipains, Plasmodium falciparum cysteine proteases as key drug targets against malaria. Curr. Med. Chem. 2011;18:1555–1572. doi: 10.2174/092986711795328328. [DOI] [PubMed] [Google Scholar]
- Tell G., Fantini D., Quadrifoglio F. Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell. Mol. Life Sci. 2010;67:3589–3608. doi: 10.1007/s00018-010-0486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari R., Straschil U., Bateman A., Bohme U., Cherevach I., Gong P., Pain A., Billker O. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8:377–387. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Wang P., Sims P.F.G., Hyde J., Cowman A. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 1998;17:3807–3815. doi: 10.1093/emboj/17.14.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dooren G.G., Su V., D’Ombrain M.C., McFadden G.I. Processing of an apicoplast leader sequence in Plasmodium falciparum and the identification of a putative leader cleavage enzyme. J. Biol. Chem. 2002;277:23612–23619. doi: 10.1074/jbc.M201748200. [DOI] [PubMed] [Google Scholar]
- Vinayak S., Sharma Y.D. Inhibition of Plasmodium falciparum ispH (lytB) gene expression by hammerhead ribozyme. Oligonucleotides. 2007;17:189–200. doi: 10.1089/oli.2007.0075. [DOI] [PubMed] [Google Scholar]
- Wang G., Shang L., Burgett A.W., Harran P.G., Wang X. Diazonamide toxins reveal an unexpected function for ornithine delta-amino transferase in mitotic cell division. Proc. Natl. Acad. Sci. USA. 2007;104:2068–2073. doi: 10.1073/pnas.0610832104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Wang Q., Aspinall T.V., Sims P.F., Hyde J.E. Transfection studies to explore essential folate metabolism and antifolate drug synergy in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2004;51:1425–1438. doi: 10.1111/j.1365-2958.2003.03915.x. [DOI] [PubMed] [Google Scholar]
- Wang P., Wang Q., Yang Y., Coward J.K., Nzila A., Sims P.F., Hyde J.E. Characterisation of the bifunctional dihydrofolate synthase-folylpolyglutamate synthase from Plasmodium falciparum; a potential novel target for antimalarial antifolate inhibition. Mol. Biochem. Parasitol. 2010;172:41–51. doi: 10.1016/j.molbiopara.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse R.M., Zdobnov E.M., Kriventseva E.V. Correlating traits of gene retention, sequence divergence, duplicability and essentiality in vertebrates, arthropods, and fungi. Genome Biol. Evol. 2010;3:75–86. doi: 10.1093/gbe/evq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells T.N., Scully P., Paravicini G., Proudfoot A.E., Payton M.A. Mechanism of irreversible inactivation of phosphomannose isomerases by silver ions and flamazine. Biochemistry. 1995;34:7896–7903. doi: 10.1021/bi00024a014. [DOI] [PubMed] [Google Scholar]
- Wills E.A., Roberts I.S., Del Poeta M., Rivera J., Casadevall A., Cox G.M., Perfect J.R. Identification and characterization of the Cryptococcus neoformans phosphomannose isomerase-encoding gene, MAN1, and its impact on pathogenicity. Mol. Microbiol. 2001;40:610–620. doi: 10.1046/j.1365-2958.2001.02401.x. [DOI] [PubMed] [Google Scholar]
- Winzeler E.A., Shoemaker D.D., Astromoff A., Liang H., Anderson K., Andre B., Bangham R., Benito R., Boeke J.D., Bussey H., Chu A.M., Connelly C., Davis K., Dietrich F., Dow S.W., El Bakkoury M., Foury F., Friend S.H., Gentalen E., Giaever G., Hegemann J.H., Jones T., Laub M., Liao H., Liebundguth N., Lockhart D.J., Lucau-Danila A., Lussier M., M’Rabet N., Menard P., Mittmann M., Pai C., Rebischung C., Revuelta J.L., Riles L., Roberts C.J., Ross-MacDonald P., Scherens B., Snyder M., Sookhai-Mahadeo S., Storms R.K., Veronneau S., Voet M., Volckaert G., Ward T.R., Wysocki R., Yen G.S., Yu K., Zimmermann K., Philippsen P., Johnston M., Davis R.W. Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science. 1999;285:901–906. doi: 10.1126/science.285.5429.901. [DOI] [PubMed] [Google Scholar]
- Wydysh E.A., Medghalchi S.M., Vadlamudi A., Townsend C.A. Design and synthesis of small molecule glycerol 3-phosphate acyltransferase inhibitors. J. Med. Chem. 2009;52:3317–3327. doi: 10.1021/jm900251a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Imamura T., Kusaba K., Shinoda S. Purification and some properties of inducible lysine decarboxylase from Vibrio parahaemolyticus. Chem. Pharm. Bull. (Tokyo) 1991;39:3067–3070. doi: 10.1248/cpb.39.3067. [DOI] [PubMed] [Google Scholar]
- Yamashita, S., Numa, S., 1981. Glycerophosphate acyltransferase from rat liver. Methods Enzymol. 71 (Pt C), pp. 550–554. [DOI] [PubMed]
- Yeh I., Hanekamp T., Tsoka S., Karp P.D., Altman R.B. Computational analysis of Plasmodium falciparum metabolism: organizing genomic information to facilitate drug discovery. Genome Res. 2004;14:917–924. doi: 10.1101/gr.2050304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zawahir Z., Dayam R., Deng J., Pereira C., Neamati N. Pharmacophore guided discovery of small-molecule human apurinic/apyrimidinic endonuclease 1 inhibitors. J. Med. Chem. 2009;52:20–32. doi: 10.1021/jm800739m. [DOI] [PubMed] [Google Scholar]
- Zhang K., Rathod P.K. Divergent regulation of dihydrofolate reductase between malaria parasite and human host. Science. 2002;296:545–547. doi: 10.1126/science.1068274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


