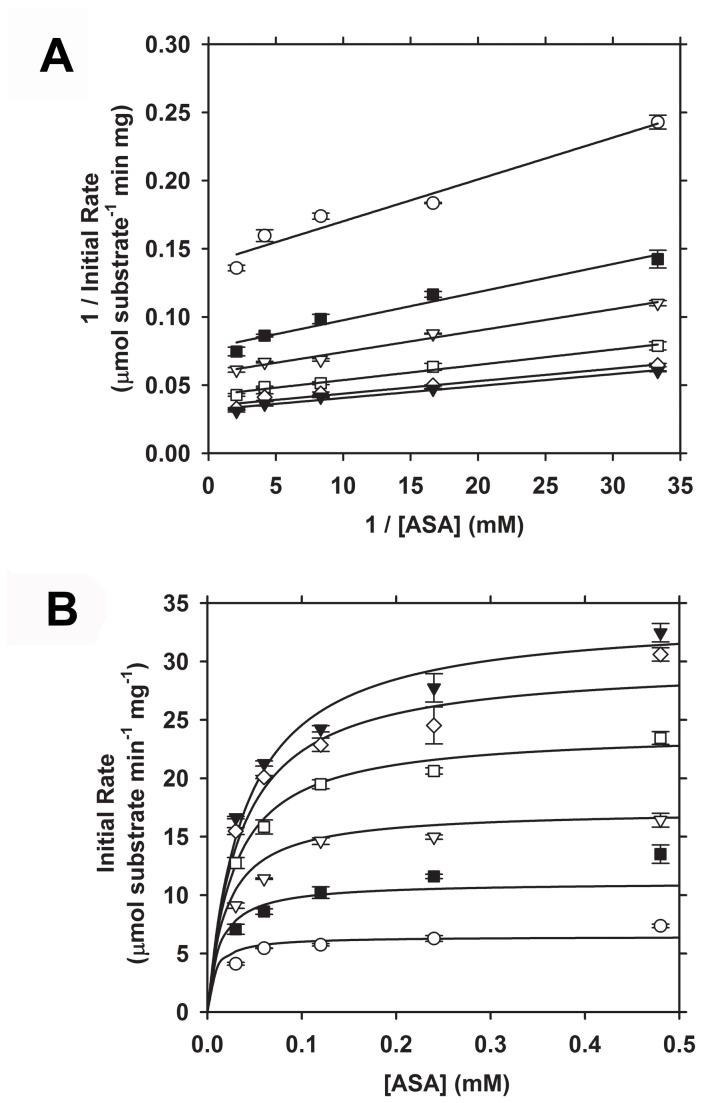
Figure 6. Enzyme kinetic profiles of recombinant Sp-DHDPS.
Initial velocity was measured as a function of (S)-ASA concentration. Experiments were conducted at fixed pyruvate concentrations of () 0. 5 mM, () 1.0 mM, () 2.0 mM, () 4.0 mM, () 8.0 mM and () 16.0 mM. (A) Lineweaver-Burk plots showing multiple parallel lines; diagnostic of a ping pong kinetic mechanism [25,67,69]. (B) Michaelis-Menten plots of data shown in A, where solid lines represent the global nonlinear best-fit to a Ping-Pong mechanism (without substrate inhibition) using ENZFITTER software, resulting in a R 2 = 0.98 and the enzyme kinetic parameters summarized in Table 5.