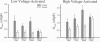Abstract
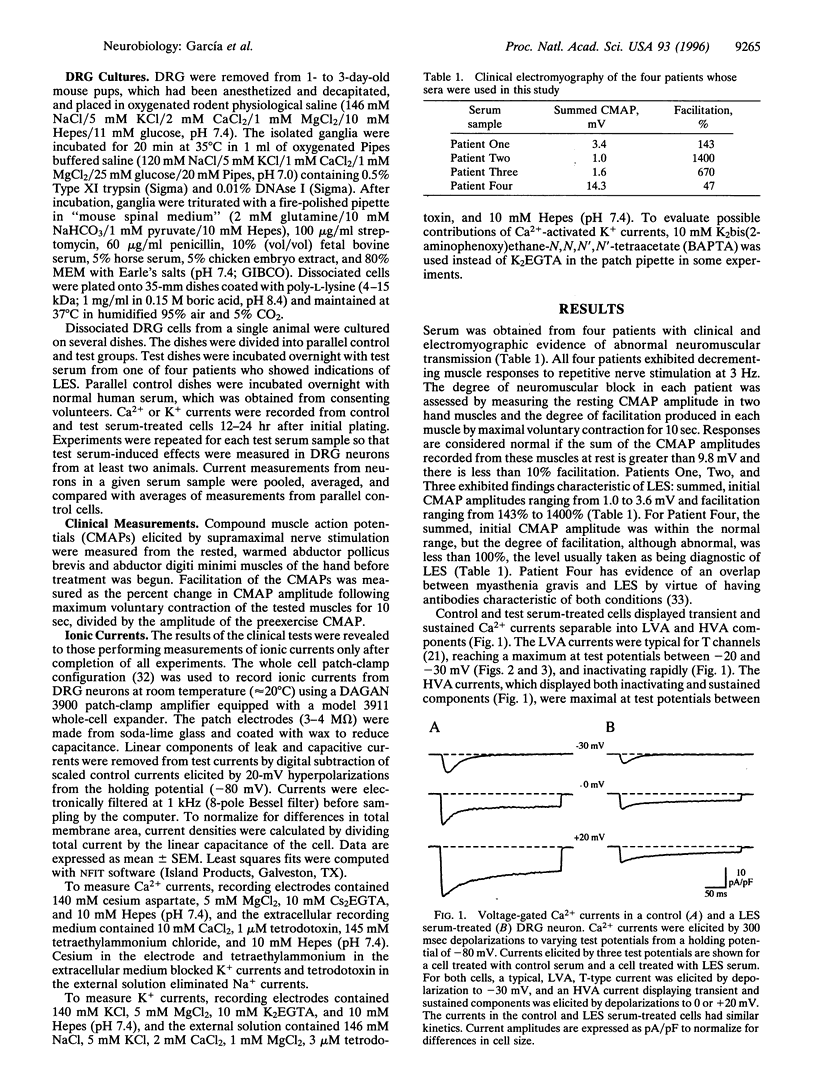
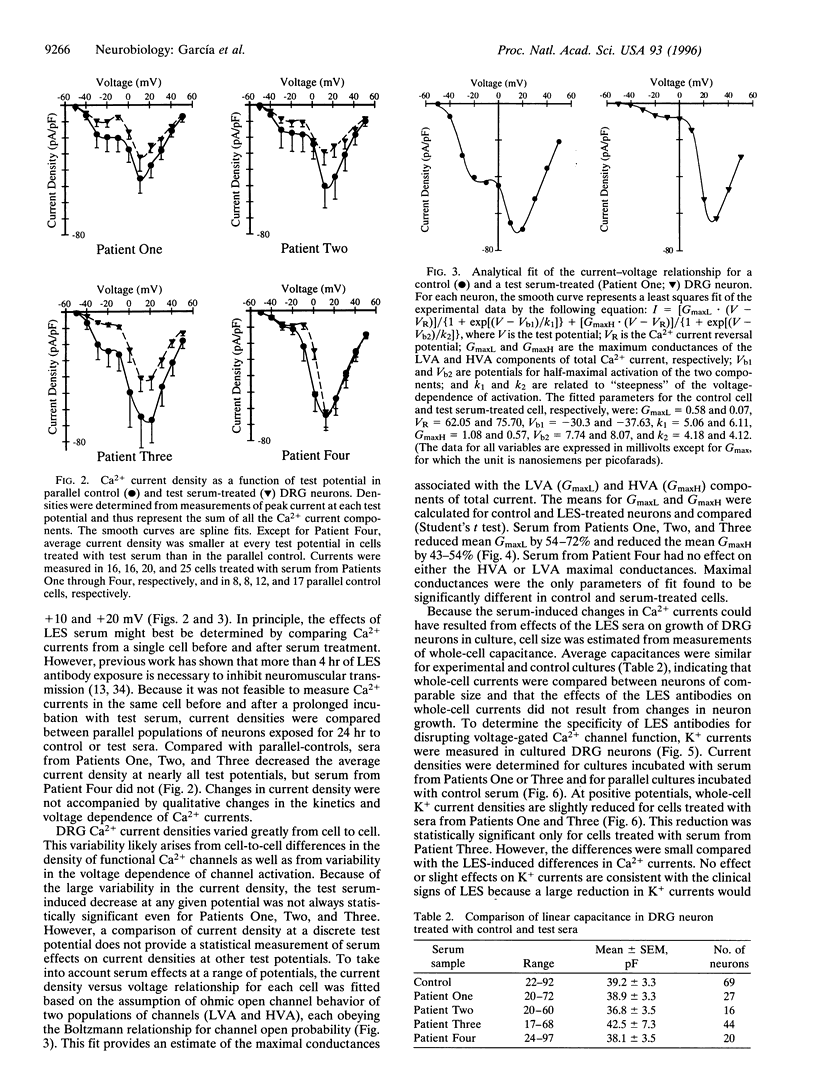
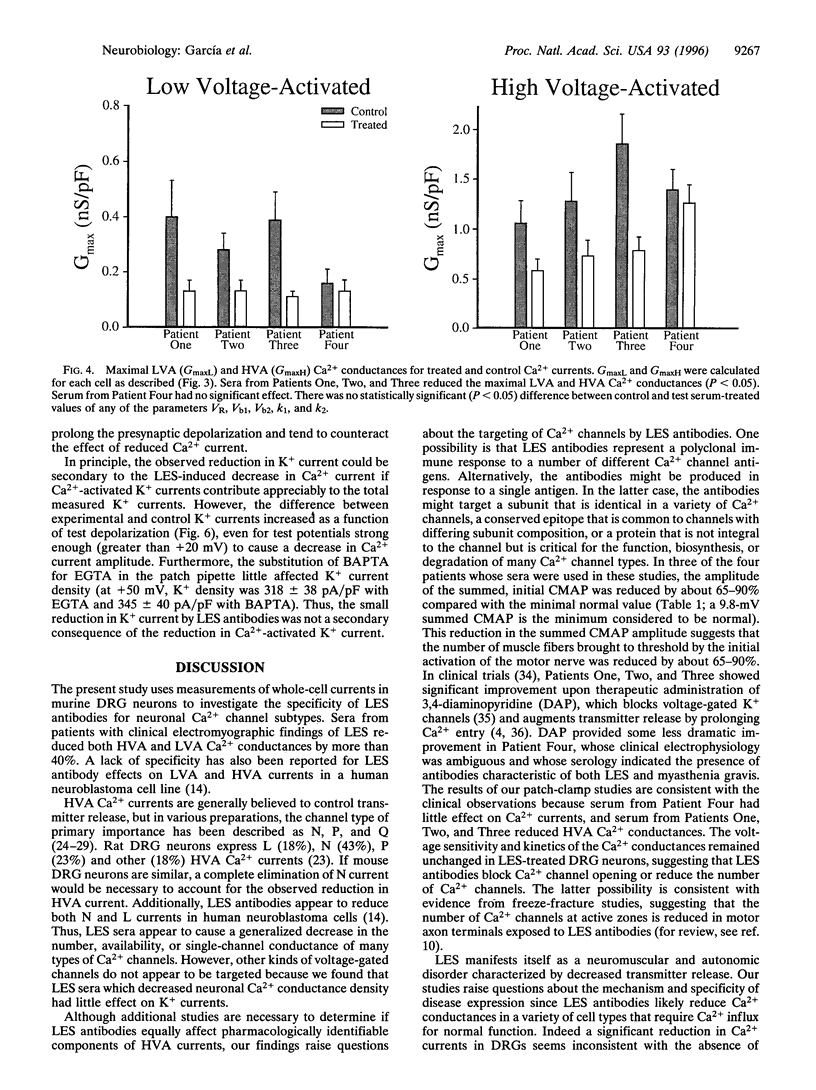
Voltage-gated Ca2+ channels are categorized as either high-voltage activated (HVA) or low-voltage activated (LVA), and a subtype (or subtypes) of HVA Ca2+ channels link the presynaptic depolarization to rapid neuro-transmitter release. Reductions in transmitter release are characteristic of the autoimmune disorder, Lambert-Eaton syndrome (LES). Because antibodies from LES patients reduce Ca2+ influx in a variety of cell types and disrupt the intramembrane organization of active zones at neuromuscular synapses, specificity of LES antibodies for the Ca2+ channels that control transmitter release has been suggested as the mechanism for disease. We tested sera from four patients with LES. Serum samples from three of the four patients reduced both the maximal LVA and HVA Ca2+ conductances in murine dorsal root ganglion neurons. Thus, even though LES is expressed as a neuromuscular and autonomic disorder, our studies suggest that Ca2+ channels may be broadly affected in LES patients. To account for the specificity of disease expression, we suggest that incapacitation of only a fraction of the Ca2+ channels clustered at active zones would severely depress transmitter release. In particular, if several Ca2+ channels in a cluster are normally required to open simultaneously before transmitter release becomes likely, the loss of a few active zone Ca2+ channels would exponentially reduce the probability of transmitter release. This model may explain why LES is expressed as a neuromuscular disorder and can account for a clinical hallmark of LES, facilitation of neuromuscular transmission produced by vigorous voluntary effort.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L. Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol. 1976;7(Pt 4):291–391. doi: 10.1016/0301-0082(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Adler E. M., Charlton M. P. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann N Y Acad Sci. 1991;635:365–381. doi: 10.1111/j.1749-6632.1991.tb36505.x. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986 Dec;381:619–640. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Charlton M. P., Smith S. J. Calcium entry and transmitter release at voltage-clamped nerve terminals of squid. J Physiol. 1985 Oct;367:163–181. doi: 10.1113/jphysiol.1985.sp015819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandino J. K., Kim Y. I. Lambert-Eaton syndrome IgG inhibits dihydropyridine-sensitive, slowly inactivating calcium channels in bovine adrenal chromaffin cells. Ann N Y Acad Sci. 1993 Jun 21;681:394–397. doi: 10.1111/j.1749-6632.1993.tb22918.x. [DOI] [PubMed] [Google Scholar]
- Blundon J. A., Wright S. N., Brodwick M. S., Bittner G. D. Residual free calcium is not responsible for facilitation of neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9388–9392. doi: 10.1073/pnas.90.20.9388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaux R., Pécot-Dechavassine M. Vésicules synaptiques et poches au niveau des "zones actives" de la jonction neuromusculaire. C R Acad Sci Hebd Seances Acad Sci D. 1970 Dec 21;271(25):2346–2349. [PubMed] [Google Scholar]
- Dodge F. A., Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967 Nov;193(2):419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer F., Peper K., Akert K., Sandri C., Moor H. Ultrastructure of the "active zone" in the frog neuromuscular junction. Brain Res. 1973 Nov 23;62(2):373–380. doi: 10.1016/0006-8993(73)90699-9. [DOI] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Lambert E. H. Detailed analysis of neuromuscular transmission in a patient with the myasthenic syndrome sometimes associated with bronchogenic carcinoma. Mayo Clin Proc. 1968 Oct;43(10):689–713. [PubMed] [Google Scholar]
- Engel A. G. Review of evidence for loss of motor nerve terminal calcium channels in Lambert-Eaton myasthenic syndrome. Ann N Y Acad Sci. 1991;635:246–258. doi: 10.1111/j.1749-6632.1991.tb36496.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga H., Engel A. G., Lang B., Newsom-Davis J., Vincent A. Passive transfer of Lambert-Eaton myasthenic syndrome with IgG from man to mouse depletes the presynaptic membrane active zones. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7636–7640. doi: 10.1073/pnas.80.24.7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuoka T., Engel A. G., Lang B., Newsom-Davis J., Prior C., Wray D. W. Lambert-Eaton myasthenic syndrome: I. Early morphological effects of IgG on the presynaptic membrane active zones. Ann Neurol. 1987 Aug;22(2):193–199. doi: 10.1002/ana.410220203. [DOI] [PubMed] [Google Scholar]
- Grassi C., Magnelli V., Carabelli V., Sher E., Carbone E. Inhibition of low- and high-threshold Ca2+ channels of human neuroblastoma IMR32 cells by Lambert-Eaton myasthenic syndrome (LEMS) IgGs. Neurosci Lett. 1994 Nov 7;181(1-2):50–56. doi: 10.1016/0304-3940(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Landis D. M. Functional changes in frog neuromuscular junctions studied with freeze-fracture. J Neurocytol. 1974 Mar;3(1):109–131. doi: 10.1007/BF01111936. [DOI] [PubMed] [Google Scholar]
- Hirning L. D., Fox A. P., McCleskey E. W., Olivera B. M., Thayer S. A., Miller R. J., Tsien R. W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988 Jan 1;239(4835):57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Estimates of quantal content during 'chemical potentiation' of transmitter release. Proc R Soc Lond B Biol Sci. 1979 Aug 31;205(1160):369–378. doi: 10.1098/rspb.1979.0070. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Blandino J. K., O'Shaughnessy T. J. Inhibitory action of Lambert-Eaton syndrome IgG on calcium currents in a thyroid C-cell line. Ann N Y Acad Sci. 1993 Jun 21;681:398–401. doi: 10.1111/j.1749-6632.1993.tb22919.x. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Neher E. IgG from patients with Lambert-Eaton syndrome blocks voltage-dependent calcium channels. Science. 1988 Jan 22;239(4838):405–408. doi: 10.1126/science.2447652. [DOI] [PubMed] [Google Scholar]
- Kim Y. I., Sanders D. B., Johns T. R., Phillips L. H., Smith R. E. Lambert-Eaton myasthenic syndrome: the lack of short-term in vitro effects of serum factors on neuromuscular transmission. J Neurol Sci. 1988 Oct;87(1):1–13. doi: 10.1016/0022-510x(88)90049-4. [DOI] [PubMed] [Google Scholar]
- Kirsch G. E., Narahashi T. 3,4-diaminopyridine. A potent new potassium channel blocker. Biophys J. 1978 Jun;22(3):507–512. doi: 10.1016/S0006-3495(78)85503-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E. H., Elmqvist D. Quantal components of end-plate potentials in the myasthenic syndrome. Ann N Y Acad Sci. 1971 Sep 15;183:183–199. doi: 10.1111/j.1749-6632.1971.tb30750.x. [DOI] [PubMed] [Google Scholar]
- Lingle C. J., Steinbach J. H. Neuromuscular blocking agents. Int Anesthesiol Clin. 1988 Winter;26(4):288–301. doi: 10.1097/00004311-198802640-00007. [DOI] [PubMed] [Google Scholar]
- Login I. S., Kim Y. I., Judd A. M., Spangelo B. L., MacLeod R. M. Immunoglobulins of Lambert-Eaton myasthenic syndrome inhibit rat pituitary hormone release. Ann Neurol. 1987 Nov;22(5):610–614. doi: 10.1002/ana.410220509. [DOI] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- O'Neill J. H., Murray N. M., Newsom-Davis J. The Lambert-Eaton myasthenic syndrome. A review of 50 cases. Brain. 1988 Jun;111(Pt 3):577–596. doi: 10.1093/brain/111.3.577. [DOI] [PubMed] [Google Scholar]
- Paton W. D., Waud D. R. The margin of safety of neuromuscular transmission. J Physiol. 1967 Jul;191(1):59–90. doi: 10.1113/jphysiol.1967.sp008237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C., Lang B., Newsom-Davis J., Wray D. W. Selective action of myasthenic syndrome antibodies on calcium channels in a rodent neuroblastoma x glioma cell line. J Physiol. 1990 Feb;421:293–308. doi: 10.1113/jphysiol.1990.sp017945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Roberts A., Perera S., Lang B., Vincent A., Newsom-Davis J. Paraneoplastic myasthenic syndrome IgG inhibits 45Ca2+ flux in a human small cell carcinoma line. Nature. 1985 Oct 24;317(6039):737–739. doi: 10.1038/317737a0. [DOI] [PubMed] [Google Scholar]
- Rossoni G., Berti F., La Maestra L., Clementi F. omega-Conotoxin GVIA binds to and blocks rat neuromuscular junction. Neurosci Lett. 1994 Aug 1;176(2):185–188. doi: 10.1016/0304-3940(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Sanders D. B., Howard J. F., Jr, Massey J. M. 3,4-Diaminopyridine in Lambert-Eaton myasthenic syndrome and myasthenia gravis. Ann N Y Acad Sci. 1993 Jun 21;681:588–590. doi: 10.1111/j.1749-6632.1993.tb22949.x. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan S., Bittner G. D., Brodwick M. S. Calcium-activated potassium conductance in presynaptic terminals at the crayfish neuromuscular junction. J Gen Physiol. 1991 Dec;98(6):1161–1179. doi: 10.1085/jgp.98.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991 Apr;11(4):985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993 Dec;11(6):1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Adams M. E., Dunlap K. Multiple Ca2+ channel types coexist to regulate synaptosomal neurotransmitter release. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9518–9522. doi: 10.1073/pnas.90.20.9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viglione M. P., Blandino J. K., Kim S. J., Kim Y. I. Effects of Lambert-Eaton syndrome serum and IgG on calcium and sodium currents in small-cell lung cancer cells. Ann N Y Acad Sci. 1993 Jun 21;681:418–421. doi: 10.1111/j.1749-6632.1993.tb22925.x. [DOI] [PubMed] [Google Scholar]
- Viglione M. P., Creutz C. E., Kim Y. I. Lambert-Eaton syndrome: antigen-antibody interaction and calcium current inhibition in chromaffin cells. Muscle Nerve. 1992 Dec;15(12):1325–1333. doi: 10.1002/mus.880151206. [DOI] [PubMed] [Google Scholar]
- Walrond J. P., Govind C. K., Huestis S. E. Two structural adaptations for regulating transmitter release at lobster neuromuscular synapses. J Neurosci. 1993 Nov;13(11):4831–4845. doi: 10.1523/JNEUROSCI.13-11-04831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrond J. P., Reese T. S. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J Neurosci. 1985 May;5(5):1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waud B. E., Waud D. R. The margin of safety of neuromuscular transmission in the muscle of the diaphragm. Anesthesiology. 1972 Oct;37(4):417–422. doi: 10.1097/00000542-197210000-00012. [DOI] [PubMed] [Google Scholar]
- Wheeler D. B., Randall A., Tsien R. W. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994 Apr 1;264(5155):107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Zucker R. S. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]
- von Gersdorff H., Matthews G. Dynamics of synaptic vesicle fusion and membrane retrieval in synaptic terminals. Nature. 1994 Feb 24;367(6465):735–739. doi: 10.1038/367735a0. [DOI] [PubMed] [Google Scholar]