Abstract
Aim
To review the treatment results and identify prognostic factors for disease control and survival in a cohort of nasopharyngeal carcinoma (NPC) patients from a non-endemic population in Slovenia, diagnosed between 1990 and 2003.
Background
In Caucasians, nasopharyngeal carcinoma is a rare malignant tumor. Its diagnosis and treatment are complex and have been dramatically impacted by recent technological advances.
Materials and methods
In the Cancer Registry of Slovenia database, a total of 126 patients with NPC were identified, 93 of whom were available for analysis. All patients were treated with conventional two-dimensional radiotherapy (RT) and 29.3% underwent chemotherapy (ChT).
Results
The median follow-up time for those alive at the last follow-up examination was 74.5 months. Disease recurred locally in 17 patients, regionally in 4 patients and at distant sites in 18 patients, resulting in 5-year locoregional control (LRC), distant failure-free survival (DFFS) and disease-free survival (DFS) of 73.7%, 78.6% and 59.3%, respectively. Disease-specific survival at 5 years was 59% and overall survival (OS) was 49.7%. In a multivariate analysis, LRC was favorably affected (P < 0.05) by an undifferentiated histology (hazard ratio [HR] = 2.86), DFFS through the absence of neck metastases (HR = 0.28), DFS by younger age (HR = 0.46), and more intensive RT (expressed as the isoeffective dose, EQD2,T; HR = 2.08). The independent prognosticator for OS was age (≤55 years vs. >55 years, HR = 0.39); in the ≤55 years subgroup, an improved OS was connected to a more intensive RT regimen of EQD2,T ≥ 66 Gy (HR = 4.17).
Conclusions
Our results confirm an independent and favorable effect from an undifferentiated histology, the absence of neck metastases, a younger patient age at diagnosis, and more intensive RT regimens for disease control and survival.
Keywords: Nasopharyngeal carcinoma, Radiotherapy, Chemotherapy, Survival, Prognostic factors
1. Background
Nasopharyngeal carcinoma (NPC) is a rare malignant tumor which appears sporadically in Slovenia and most parts of the Western world.1 According to the National Cancer Registry of Slovenia, during the period 1990–2003 the annual incidence of nasopharyngeal cancer was 0.75/100,000 males and 0.32/100,000 females.2 Multiple etiological factors were suggested to contribute to the increased incidence of the disease, including diet, viral agents and genetic susceptibility. The symptoms and signs of nasopharyngeal carcinoma are non-specific; furthermore, the nasal part of the pharynx is difficult to access for an unskilled examiner, which is why a substantial proportion of the patients at the time of diagnosis already displayed advanced stages of the disease.1
Modern imaging techniques, including computer tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) allow for the recognition of disease extensions that are not visible at clinical examination. They have dramatically impacted the radiotherapy (RT) procedures and techniques used in NPC which is the main treatment modality for this disease. Chemotherapy (ChT) has a role to play in advanced stages of the disease, whereas up-front surgery is rarely used in NPC due to the complexity of the anatomical relationship with adjacent structures and related morbidity.3
2. Aim
The majority of studies carried out on NPC have been conducted in endemic countries. Therefore, the present analysis of the retrospective series of NPC patients diagnosed and treated in Slovenia between 1990 and 2003 may highlight the range of conventional two-dimensional RT in the treatment of this rare disease in non-endemic areas. The secondary aim of the study was to identify prognostic factors for disease control and survival.
3. Materials and methods
3.1. Patient and disease characteristics
Between 1990 and 2003, a total of 126 patients with NPC were registered in the National Cancer Registry database.2 Patients less than 16 years of age (who entered pediatric treatment protocols, 5), those with no therapy or treated with palliative intent (15), patients with a primary tumor originating outside the nasopharynx (found in the clinical chart review, 9), and those with incomplete documentation (3) or recurrent disease (1) were excluded. A total of 93 patients treated with curative intent left for detailed analysis. In this group, the median age and the ratio between sexes was 55 years (average 53.8 years, range 20–86 years) and 2.3:1 (65 males vs. 28 females), respectively. Details on the characteristics of the patients and tumors are shown in Table 1.
Table 1.
Characteristics of patients and their tumors.
| Patients (N = 93) | |
| Sex (female/male) | 28/65 |
| Age | 55 (20–86)a |
| UICC TNM-classification (2002) | N0 | N1 | N2 | N3 | Total |
|---|---|---|---|---|---|
| TX | 0 | 1 | 0 | 0 | 1 |
| T1 | 6 | 12 | 10 | 6 | 34 |
| T2 | 8 | 9 | 3 | 1 | 21 |
| T3 | 7 | 0 | 3 | 1 | 11 |
| T4 | 15 | 4 | 2 | 5 | 26 |
| Total | 36 | 26 | 18 | 13 | 93 |
| Overall stage | |
| Stage I | 6 (6.5%) |
| Stage IIA | 6 (6.5%) |
| Stage IIB | 23 (24.7) |
| Stage III | 23 (24.7) |
| Stage IVA | 21 (22.6%) |
| Stage IVB | 14 (15.1%) |
| Histology | |
| WHO I | 4 (4.3%) |
| WHO II | 20 (21.5%) |
| WHO III | 68 (73.1%) |
| Other | 1 (1.1%) |
| Diagnostic CT/MRI | 71 (76.3%) |
| Chemotherapy | 21 (22.6%) |
UICC, International Union Against Cancer; WHO, World Health Organization; CT, computed tomography; MR, magnetic resonance.
Median (range), in years.
The median time to elapse from the onset of the first symptom or sign to the histological confirmation of the disease was 3 months (range 0–24 months): this interval was 3–6 months in 24 patients (25.8%), 6–12 months in 13 (14%) and over a year in 6 patients (6.5%). The most common symptom was a palpable mass in the neck (48.4%), followed by nasal obstruction or a persistent/bloody nasal discharge (18.3%), hearing loss or otalgia (10.8%), headache (9.7%), neurological (7.5%) and pharyngeal symptoms (sore throat, dysphagia; 2.2%). In three patients (3.2%), the disease was discovered by chance. Upon initial examination, cranial nerve involvement was found in 20 patients (21.5%). The most frequently involved cranial nerve was n.V (14), followed by n.VI (6), nn.III, IX and X (in 5 patients each), n.XII (4), n.VII (3), n.IV (2) and n.VIII (1). Ten patients had isolated nerve dysfunction and ten patients had multiple cranial nerve involvement.
All patients underwent an endoscopy with a biopsy, a complete blood count and blood chemistry profiles with liver and renal function tests, a chest X-ray or CT, and ultrasonography of the abdomen, whereas other examinations (e.g. a bone scan) were conducted if clinically indicated. A pre-treatment diagnostic CT, MRI or both of the nasopharynx and the neck were carried out in 68 (73.1%), 1 and 2 of the patients, respectively. PET was not yet available in Slovenia at that time. Using the World Health Organization's (WHO) histological classification of NPC,4 68 (73.1%) patients had undifferentiated carcinoma (WHO type III), whereas nonkeratinizing carcinoma (WHO type II) and squamous cell carcinoma (WHO type I) were present in 20 (21.5%) and 4 (4.3%) patients, respectively; one patient had spindle cell carcinoma. All patients were retrospectively re-staged for the purpose of the present study according to the International Union Against Cancer (UICC) 2002 staging system.5 As a result, the majority of patients were found to have advanced-stage disease: I – 6 (6.5%), IIA – 6 (6.5%), IIB – 23 (24.7%), III – 23 (24.7%), IVA – 21 (22.6%) and IVB – 14 (15.1%).
3.2. Treatment
3.2.1. Radiotherapy
All patients were irradiated with cobalt-60 (46, 49.5%) or linear accelerator (47, 50.5%) 5–8 MV photon beams and a conventional 3-field technique using two-dimensional computer planning, head holders with thermoplastic casts and individual shielding blocks. The median daily dose was 2 Gy (range 1.8–3 Gy; >2 Gy in 3 patients) applied in one fraction, five times per week, as specified on the 100% isodose curve. The median total dose to the gross tumor volume was 70 Gy (range 51–75.5 Gy, >60 Gy in 95.7%). The gross tumor volume and a margin of 1.5–2 cm was within at least the 95% isodose curve and, to achieve dose homogeneity within a range of ±5% inside the treated volume, wedge filters and tissue compensation boluses were used when appropriate. Clinically uninvolved lymphatic drainage basins on the neck received 50 Gy and the spinal cord was shielded after a dose of 46 Gy. After off-cord photon field reduction, the dose to the posterior lymph node triangle was supplemented with electron beams of appropriate energy (9–12 MeV) to the prescribed dose level. Portal films were obtained at the beginning of the RT course and whenever a change of technique occurred.
Since the overall treatment time differed significantly among the patients (median 53 days, range 40–84 days), the isoeffective dose (EQD2,T) was calculated to compare the intensity of the different radiotherapy regimens employed using the following formula:
where EQD2 is the equivalent total dose in 2 Gy fractions (Gy); T is the actual overall treatment time (in days); t is the expected overall treatment time (47 days); Dprolif is the dose recovered per day due to proliferation (0.7 Gy/day).6 When considering the actual duration of the RT course in our patients, the median EQD2,T was 65 Gy (range 47–71.4 Gy; >60 Gy in 74.2%).
3.2.2. Chemotherapy
In 21 (22.6%) patients, chemotherapeutics were administered concurrently with RT. During RT, all but one patient were administered cisplatin (100 mg/m2 I.V. in 3-week intervals) which was combined in one patient with 5-fluorouracil (1000 mg/m2 I.V. days 1–4). One patient had a combination of mitomycin C (15 mg/m2 I.V. at 10 Gy of RT) and bleomycin (5 mg I.M. twice per week). The median number of concurrent ChT administrations was 2 (range 1–3 cycles).
Adjuvantly, various combinations of cisplatin (100 mg/m2, day 1), carboplatin (AUC 6, day 1) and 5-fluorouracil (1000 mg/m2, days 1–5) were administered at 3-week intervals in 7 (7.5%) patients (all also underwent concomitant ChT). The median number of adjuvant ChT cycles was 3 (range 1–3).
3.2.3. Surgery
Seven patients (7.5%) underwent surgical resection. One patient had a partial resection of the primary tumor for diagnostic purposes. Prior to initiation and after completion of the RT (for residual disease), a neck dissection was carried out in two and four patients, respectively. In the latter group, malignant cells were found in two out of the four cases.
3.3. Statistics
The statistical analysis was performed using the PC SPSS (Release 13.0, SPSS Inc., Chicago, IL) statistical package. A univariate analysis of patients’ survival was carried out using the Kaplan–Meier product-limit method with 95% confidence intervals (CI) reported7 and the differences between potential prognostic subgroups were tested for significance using the log-rank test.8 To identify independent prognostic factors for disease control and survival, a multivariate analysis was performed with the Cox regression model.9 All of the tests were two-sided, and the results were considered significant at a probability level of ≤5%.
Survival times were calculated from the date of histological confirmation of the disease. Persistence of the disease for more than three months post-therapy or progression following a complete response after (ChT)RT or unsuccessful surgery (if performed) with residual disease left behind and distant metastases was defined as a failure. The endpoints of the survival analysis were local (LC), regional (RC) and locoregional control (LRC) (persistent disease or recurrence in the nasopharynx, in the neck or in both sites, respectively, is considered as an event), distant failure-free survival (DFFS, the appearance of systemic metastases considered as an event), disease-free survival (DFS, persistent/recurrent disease locally, regionally and/or at distant sites considered as an event), disease-specific survival (DSS, death due to disease considered as an event), and overall survival (OS, death of whatever cause considered as an event).
4. Results
The median follow-up time for all patients was 38 months (range 1–181 months) and was 74.5 months (range 5–181 months) in those alive at the most recent follow-up examination.
4.1. Pattern of treatment failure
Treatment failed locally in 17 (18.3%) patients within an interval of 0–35 months (median 12 months). LC at 5 years was 78.4% (95% CI 69.1–87.6). Four patients failed in the neck, all with primary controlled, 5–14 months (median 9.5 months) after diagnosing NPC, resulting in RC at 5 years of 95% (95% CI 90.3–99.8). The LRC at 5 years was 73.7% (95% CI 63.9–83.5) (Fig. 1).
Fig. 1.
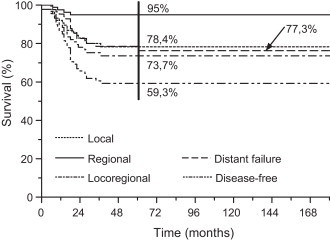
Local, regional and distant control.
A total of 18 patients (19.3%) developed distant metastases, resulting in a 5-year DFFS of 78.6% (95% CI 69.5–87.7) (Fig. 1). The median time for the occurrence of systemic disease was 19.8 months (range 6–60 months; ≤3 years in 16 patients) with the most frequently affected sites being the bone (10), liver (3) and lymph nodes below the clavicles (3), followed by lung and subcutis (in one patient each). Distant metastases were the first sign of disease progression in 13 patients, whereas 5 patients experienced distant failure after regional (3) or local failure (2). The DFS at 5 years for all 93 patients was 59.3% (95% CI 48.7–69.9) (Fig. 1).
Upon a multivariate analysis, only histology was identified as an independent prognostic factor for LRC with an undifferentiated variant favorably affecting prognosis when compared to nonkeratinizing tumors (p = 0.02, hazard ratio [HR] = 2.68, 95% CI 1.16–7.14). With regard to DFFS, N0-neck was confirmed as an independent and positive prognosticator (N0 vs. N+: p = 0.045, HR = 0.28, 95% CI 0.08–0.97). DFS was found to be better for younger patients (≤55 years vs. >55 years: p = 0.027, HR = 0.46, 95% CI 0.23–0.92) and for those irradiated with higher EQD2,T (<64 Gy vs. ≥64 Gy: p = 0.037, HR = 2.08, 95% CI 1.04–4.17) (Table 2). In patients aged ≤55 years, a higher EQD2,T (<64 Gy vs. ≥64 Gy: p = 0.035, HR = 3.45, 95% CI 1.09–11.11) and undifferentiated histology (nonkeratinizing vs. undifferentiated: p = 0.002, HR = 5.55, 95% CI 1.82–16.67) favorably affected DFS in a multivariate analysis (Fig. 2A).
Table 2.
Results of multivariate analysis for various endpoints.
| Endpoint | Independent prognostic factor(s) | p-Value | HR (95% CI) |
|---|---|---|---|
| LRC | Histology: WHO type II vs. III | 0.02 | 2.86 (1.16–7.14) |
| DFFS | N stage: N0 vs. N+ | 0.045 | 0.28 (0.08–0.97) |
| DFS | Age: ≤55 years vs. >55 years | 0.027 | 0.46 (0.23–0.92) |
| EQD2,T: <64 Gy vs. ≥64 Gy | 0.037 | 2.08 (1.04–4.17) | |
| OS | Age (years): ≤ 55 vs. >55 | 0.001 | 0.39 (0.23–0.68) |
LRC, loco-regional control; DFFS, distant failure-free survival; DFS, disease-free survival; OS, overall survival; EQD2,T, equivalent tumor dose; HR, hazard ratio; CI, confidence interval.
Fig. 2.
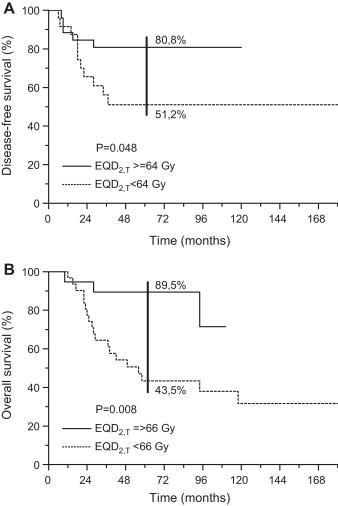
Patients ≤55 years of age: effect of intensity of radiotherapy (EQD2,T): A, disease-free survival; B, overall survival.
4.2. Salvage therapy and outcome
A total of 13 out of the 17 (76.5%) patients with local failure and 3 out of the 4 patients with regional failure had salvage therapy. Tele-RT was used in 12 locally recurrent cases and adjuvant to surgery in one case with a neck recurrence (the other two patients had neck dissections); one patient with local failure had multi-agent platinum-based chemotherapy (Fig. 3). The median EQD2 of the salvage tele-RT was 30.8 Gy (range 24.8–56 Gy). In three patients with local failure who had tele-RT combined with Ir-192 low-dose-rate brachytherapy application, the cumulative re-treatment doses were 44.6, 46.5 and 63.2 Gy with a complete response achieved in only one case.
Fig. 3.
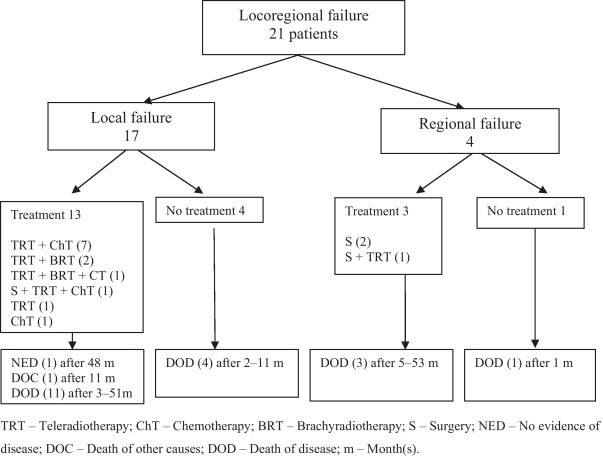
Salvage therapy for disease recurrence and outcome.
In re-treated patients, the median survival from the diagnosis of local or regional failure was 20 months (range 3–51 months) and 29 months (range 5–53 months). All but two of the patients died of disease progression. One patient was still alive 48 months after re-treatment and displayed no evidence of the disease; the other died due to secondary primary lung carcinoma but was free of NPC 11 months after salvage therapy; both had recurrences at the primary site. The median survival of the five untreated patients was five months (range 1–11 months); four of the patients died due to the progression of NPC, and the fifth one died of a new primary liver carcinoma.
A total of 16 out of 18 (88.9%) patients with distant metastases had additional therapy: z had a combination of RT and ChT, 5 patients had only ChT and 4 patients only RT. The median survival after the occurrence of systemic disease was 5 months (range 0–15 months).
4.3. Disease-specific and overall survival
On the close-out date, a total of 38 (40.9%) patients were alive with no signs of NPC and 55 (59.1%) patients died: 36 (38.7%) died of NPC and 19 (20.4%) due to causes unrelated to the disease. A secondary primary tumor developed in 14 (15.1%) patients between a 0- and 53-month (median 20 months) post-diagnosis of index NPC.
The 5-year DSS was 59% (95% CI 48.2–69.8) (Fig. 4). In a univariate analysis, a statistically significant effect of EQD2,T was observed in a subgroup of patients aged ≤55 years (<66 Gy vs. ≥66 Gy: 55.4% vs. 89.5%, p = 0.026) but was not confirmed in multivariate testing (p = 0.075, HR = 0.25, 95% CI 0.05–1.15).
Fig. 4.
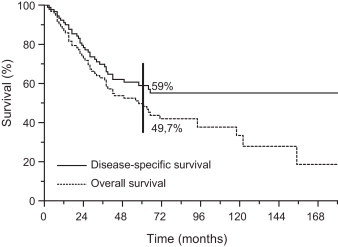
Disease-specific and overall survival.
The OS at 5 years was 49.7% (95% CI 39.2–60.2) (Fig. 4). After testing using the Cox model, a younger age was identified as prognostically advantageous (≤55 years vs. >55 years; p = 0.001, HR = 0.39, 95% CI 0.23–0.68) (Table 2). In the latter group, higher EQD2,T (≥66 Gy) emerged as an independent and favorable prognosticator over less intensive RT regimens (EQD2,T < 66 Gy; p = 0.029, HR = 4.17, 95% CI 1.15–14.29) (Fig. 2B).
4.4. Toxicity
Owing to the retrospective character of the present analysis, a robust system was used for documentation of the treatment-related complications collected from patients’ charts.
During concurrent ChT-RT with cisplatin, a transient paresis of the peroneal nerve with foot drop occurred in one patient. After therapy, there were 9/93 (.7%) cases of serious side effects documented: dysfunction of the left cranial n.XII (EQD2,T 5.6 Gy); retinopathy and optic nerve atrophy (EQD2,T 67.9 Gy with cisplatin-based concomitant ChT and one cycle of adjuvant ChT); temporal lobe injury (EQD2,T 68 Gy, 70 Gy and 127.3 Gy combined with concomitant and adjuvant ChT in two cases); necrotizing fasciitis (EQD2 124 Gy with concomitant ChT during re-treatment); hearing loss (in 3 patients, EQD2,T 63 Gy, 64.4 Gy and 69.1 Gy in combination with concomitant cisplatin: unilateral anacusis, bilateral conductive and sensorineural hypoacusis, sensorineural on the right and combined impairment on the left).
5. Discussion
When comparing the treatment results in NPC among different series reported in the literature, one should be aware of (at least) three important factors: the geographical origin of the population studied (the West vs. Asia), the time period under evaluation (owing to the evolution of diagnostic and therapeutic procedures over time), and whether the results were generated in a retrospective, pattern of practice analysis, or prospective/randomized study.1 The cohort of patients presented was found to be representative in terms of their age, sex and histological distribution when compared to other reports from Western countries, with the exception of a somewhat different ratio between squamous cell carcinoma (4.3%; expected to be up to 25%) and nonkeratinizing carcinoma (21.5%; expected to be approximately 10%).4,10 In contrast, the median age of patients reported in the Asian series is 5 years lower and almost all had undifferentiated tumors.4,11–14
The most common symptom in our patients was a palpable mass in the neck (48.4%) and the majority (62.4%) had stage III–IV disease at the time of diagnosis, with cranial nerve palsies recorded in 21.5%. These figures agree well with the literature data (10, 12, 15; Table 3). However, only 6.5% of the patients were diagnosed with a primary tumor confined to nasopharynx (stage T1) which pointed to the suboptimal use of modern imaging techniques in diagnostics (76.3% of patients had CT/MRI) and, as a result, too high a probability of an inaccurately determined T-stage of the disease. Moreover, in the early-stage disease group, the portion of radiologically staged patients was significantly lower than in the group with advanced disease (54.3% vs. 89.3%; p = 0.0003, Fisher's exact test). Compared to similar surveys conducted after the 1990s with a diagnostic CT/MRI performed in virtually all the patients,11,12,14,16 in our cohort pre-treatment evaluation was suboptimal.
Table 3.
Presenting symptoms and signs in patents with nasopharyngeal carcinoma reported in different studies.
| Author (Ref.) | Cervical mass (%) |
Cranial nerve palsy (%) | Nasal symptoms (%) | Ear symptoms (%) | |
|---|---|---|---|---|---|
| Symptom | Signa | ||||
| Sanguineti et al. 10 | 41 | 79 | 8 | 21 | 27 |
| Yi et al. 12 | 40.5 | 82.3 | 19.8 | 29 | 17 |
| Hoppe et al. 15 | 44 | 71 | 12 | 29 | 28 |
| Present study | 48.4 | 61.3 | 21.5 | 18.3 | 10.8 |
Clinical sign after physical and imaging examination.
All our patients were treated with conventional 2D-RT and with doses (the median EQD2,T was 65 Gy) usually implemented in non-endemic areas. More intensive RT with the administration of a brachy- or tele-RT boost resulting in cumulative doses well above 70 Gy was characteristic of RT practice in Asian countries11–14,16 but also in some Western RT centers.17,18 Nowadays, ChT is an integral component of all curative treatment strategies in locoregionally advanced NPC, and was administered in less than a quarter of our patients (22.6%). Specifically, in the stage III-IV subgroup this percentage was 29.3 and before 1998, when a benchmark Intergroup study 0099 on the value of adding ChT to RT was published,19 only three patients from our series received ChT. In a comparison of the results on disease control and survival across different studies from endemic and non-endemic regions where 2D-RT was used,11–14,16,20 covering approximately the same time span as that for our cohort, no striking differences with our results were observed (Table 4). Somewhat superior figures for the selected endpoints seen in the individual series should be valued in view of the lower median age of patients treated,11–14,20 higher fraction of RT-sensitive undifferentiated tumors,12,14,20 a more consistent use of CT/MRI in tumor staging11–14,16 or ChT,20 and, in particular, higher cumulative RT doses11–14,16 and the use of 3D-conformal RT techniques, including intensity-modulated RT (IMRT), in a larger proportion of patients.14 Considering the retrospective nature of these reports, including ours, it is very likely that the incidence of treatment-related late toxicity recorded has been underestimated and any detailed comparison of the results on toxicity across different studies is questionable due to various follow-up durations and detection systems implemented.
Table 4.
Two-dimensional radiotherapy technique in the treatment of nasopharyngeal carcinoma: results of disease control and survival at 5 years in studies conducted after 1990.
| Center (Ref.) | Period | N | Median age (years) | Histology grade, WHO I/II/III (%) | Diagnostic CT/MR (%) | ChT (%) | RT boost (%) | LC (%) | RC (%) | DFFS (%) | DFS (%) | DSS (%) | OS (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kaohsiung, Taiwan11 | 1983–1998 | 849 | 49 | 0.7/62.4/36.9 | 100/− | 0 | Yes (45.6) | 78 | 93.7 | 74.7 | 52 | n.s. | 59 |
| Beijing, China12 | 1990–1999 | 905 | 48 | 0.2/2.3/92.8 | All, CT ± MR | 0 | Yes (49.2) | n.s. | n.s. | n.s. | 58.4 | n.s. | 76.1 |
| Milan, Italy20 | 1990–1999 | 171 | 44 | 18 (I + II)/82 | 94/21 | 62 | No | 84 | 80 | 83 | 62 | 74 | 72 |
| Singapore13 | 1992–1994 | 677 | 46,6 | n.s. | 100/− | 0 | Yes (n.s.) | n.s. | n.s. | n.s. | n.s. | n.s. | 56.6 |
| Hong Kong, China14 a | 1996–2000 | 2687 | 47 | 0.4/99.6 (II + III) | 68/32 | 23 | Yes (55) | 85 | 94 | 81 | 63 | 80 | 75 |
| Guangzhou, China16 | 2003–2006 | 764 | n.s. | 0.7/99.1 (II + III) | −/100 | 78.4 | Yes (22.9) | 86.8 | 95.5 | 82.6 | 71.4 | n.s. | n.s. |
| Ljubljana, Slovenia | 1990–2003 | 93 | 55 | 4.3/21.5/73.1 | 73.1/3.2 | 22.6 | No | 78.4 | 95 | 78.6 | 59.3 | 59 | 49.7 |
N, number of patients; CT, computed tomography; MR, magnetic resonance; ChT, chemotherapy; RT, radiotherapy; LC, local control; RC, regional control; DFFS, distant failure-free survival; DFS, disease-free survival; DSS, disease-specific survival; OS, overall survival; n.s., not specified.
In this series, 10% of patients were treated with three-dimensional conformal or intensity-modulated radiotherapy technique.
In the present series, the beneficial effect of a younger age at diagnosis, an undifferentiated histology, the absence of neck metastases, and more intensive RT regimens for disease control and survival were observed in a multivariate analysis, which is in line with other reports.10–14,16,20 Surprisingly, we could not confirm a prognostic significance of modern imaging techniques, T-stage, and ChT for any of the endpoints studied. As the distribution of patients with CT/MRI at diagnosis differed significantly between early and advanced stages of the disease (T1, 54.3%; T2–4, 89.7%; p = 0.0003, Fisher's exact test), some patients from the early-stage group were very likely to have more advanced tumors. In addition, the risk of geographical error is also increased if modern imaging is not implemented consistently for the purpose of radiotherapy planning, which resulted in control rates and survival as poor as in the advanced-stage group. It is likely that the understaging of some of the tumors led to the lack of prognostic significance of T-stage and ChT administration seen among our patients. In fact, ChT was given more frequently to patients with more advanced tumors (stage I–II, 11.4%; stage II–IV, 29.3%; p = 0.046) although the proportion of combined treatments in this group was also fairly low. Another reason may be the low number of tumors of squamous cell histology which should, according to the meta-analysis of Bujaut et al.,21 benefit most from ChT.
What can we expect from a more consistent use of modern diagnostic imaging and systemic therapy, and from the implementation of 3D conformal RT techniques? An evaluation of the locoregional extent of the disease with MRI was recognized as an independent prognostic factor for the local control and survival of NPC patients from an endemic area of Hong Kong.14 MRI was found to cause dramatic T- and clinical stage migrations (in about 50% and 40% of cases, respectively) when compared to CT-based staging that significantly impacted the choice of treatment strategies and the tumor target delineation for RT.22,23 With 3D-planning, a highly conformal dose distribution with steep dose gradients is created, clearly superior to 2D-planning; further improvement in the locoregional control of the disease and toxicity profile (including xerostomia and quality of life testing) has been demonstrated with IMRT in a number of dosimetric studies and confirmed in retrospective comparisons of RT techniques.16,24,25 Escalating the dose to the primary by endocavitary brachytherapy or stereotactic boost may further improve local control, although at the expense of high incidence of late morbidity.11,12,17,18,26 Recently, the beneficial effects of ChT administration, preferably concomitantly with irradiation, were confirmed in several meta-analyses.21,27
6. Conclusions
The results presented confirm the efficacy (albeit far from optimal) of conventional 2D-RT in curative treatment of NPC patients from non-endemic areas, and an independent and favorable effect of undifferentiated histology, absence of neck metastases and younger patient age at diagnosis, and more intensive RT regimens for disease control and survival. They represent a good starting point when added value of modern diagnostic and treatment methods, including ChT and IMRT, is to be evaluated.
Conflict of interest
There is no conflict of interest in any of the authors of this manuscript.
Acknowledgement
Supported by the Slovenian Research Agency Grant P3-0307.
References
- 1.Hu K.S., Chan A.T.C., Costantino P., Harrison L.B. Cancer of the nasopharynx. In: Harrison L.B., Sessions R.B., Hong W.K., editors. Head and neck cancer: a multidisciplinary approach. 3rd ed. Williams & Wilkins/Wolters Kluwer; Lippincott/Philadelphia: 2009. pp. 502–529. [Google Scholar]
- 2.Cancer Registry of Slovenia. Cancer in Slovenia 1990–2003. Reports no. 32-45. Ljubljana: Institute of Oncology & Cancer Registry of Republic of Slovenia; 1993–2006.
- 3.Chan A.T.C., Felip E., on behalf of the ESMO guidelines working group Nasopharyngeal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20(Suppl. 4):iv123–iv125. doi: 10.1093/annonc/mdp150. [DOI] [PubMed] [Google Scholar]
- 4.Chan J.K.C., Bray F., McCarron P. Nasopharyngeal carcinoma. In: Barnes L., Eveson J.W., Reichart P., Sidransky D., editors. Pathology and genetics of head and neck tumors. IARC Press; Lyon: 2005. pp. 81–97. [Google Scholar]
- 5.Sobin L.H., Wittekind C., editors. TNM classification of malignant tumours. 6th ed. Wiley Liss; New York: 2002. pp. 27–33. [Google Scholar]
- 6.Bentzen S.M., Baumann M. The linear-quadratic model in clinical practice. In: Steel G.G., editor. Basic clinical radiobiology. 3rd ed. Arnold; London: 2002. pp. 134–146. [Google Scholar]
- 7.Kaplan E.L., Meir P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 8.Peto R., Pike M.C., Armitage P. Design and analysis of randomized clinical trials requiring prolong observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox D.R. Regression models and life tables. J R Stat Soc. 1972;187:187–220. [Google Scholar]
- 10.Sanguineti G., Geara F.B., Garden A.S. Carcinoma of the nasopharynx treated by radiotherapy alone: determinants of local and regional control. Int J Radiat Oncol Biol Phys. 1997;37:985–996. doi: 10.1016/s0360-3016(97)00104-1. [DOI] [PubMed] [Google Scholar]
- 11.Yeh S.A., Tang Y., Lui C.C., Huang Y.J., Huang E.Y. Treatment outcomes and late complications of 849 patients with nasopharyngeal carcinoma treated with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2005;62:672–679. doi: 10.1016/j.ijrobp.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Yi J.L., Gao L., Huang X.D. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006;65:161–168. doi: 10.1016/j.ijrobp.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Heng D.M.K., Wee J., Fong K.-W. Prognostic factors in 677 patients in Singapore with nondisseminated nasopharyngeal carcinoma. Cancer. 1999;86:1912–1920. [PubMed] [Google Scholar]
- 14.Lee A.W.M., Sze W.M., Au J.S.K. Treatment results for nasopharyngeal carcinoma in the modern era: the Hong Kong experience. Int J Radiat Oncol Biol Phys. 2005;61:1107–1116. doi: 10.1016/j.ijrobp.2004.07.702. [DOI] [PubMed] [Google Scholar]
- 15.Hoppe R.T., Goffinet D.R., Bagshaw M.A. Carcinoma of the nasopharynx. Eighteen years’ experience with megavoltage radiation therapy. Cancer. 1976;37:2605–2612. doi: 10.1002/1097-0142(197606)37:6<2605::aid-cncr2820370607>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 16.Lai S.-Z., Li W.-F., Chen L. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int J Radiat Oncol Biol Phys. 2011;80:661–668. doi: 10.1016/j.ijrobp.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Hara W., Loo B.W., Goffinet D.R. Excellent local control with stereotactic radiotherapy boost after external beam radiotherapy in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2008;71:393–400. doi: 10.1016/j.ijrobp.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Schinagl D.A.X., Marres H.A.M., Kappelle A.C. External beam radiotherapy with endocavitary boost for nasopharyngeal cancer: treatment results and late toxicity after extended follow-up. Int J Radiat Oncol Biol Phys. 2010;78:689–695. doi: 10.1016/j.ijrobp.2009.08.072. [DOI] [PubMed] [Google Scholar]
- 19.Al-Sarraf M., LeBlanc M., Giri P.G.S. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099. J Clin Oncol. 1998;16:1310–1317. doi: 10.1200/JCO.1998.16.4.1310. [DOI] [PubMed] [Google Scholar]
- 20.Palazzi M., Guzzo M., Tomatis S. Improved outcome of nasopharyngeal carcinoma treated with conventional radiotherapy. Int J Radiat Oncol Biol Phys. 2004;60:1451–1458. doi: 10.1016/j.ijrobp.2004.05.059. [DOI] [PubMed] [Google Scholar]
- 21.Baujat B., Audry H., Bourhis J. Chemotherapy in locally advanced nasopharyngeal carcinoma: an individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol Biol Phys. 2006;64:47–56. doi: 10.1016/j.ijrobp.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 22.Liao X.-B., Mao Y.-P., Liu L.-Z. How does magnetic resonance imaging influence staging according to AJCC staging system for nasopharyngeal carcinoma compared with computed tomography. Int J Radiat Oncol Biol Phys. 2008;72:1368–1377. doi: 10.1016/j.ijrobp.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 23.Trojanowska A. Squamous cell carcinoma of the head and neck – the role of diffusion and perfusion imaging in tumor recurrence and follow up. Rep Pract Oncol Radiother. 2011;16:203–208. doi: 10.1016/j.rpor.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palazzi M., Orlandi E., Bossi P. Further improvement in outcomes of nasopharyngeal carcinoma with optimized radiotherapy and induction plus concomitant chemotherapy: an update of the Milan experience. Int J Radiat Oncol Biol Phys. 2009;74:774–780. doi: 10.1016/j.ijrobp.2008.08.068. [DOI] [PubMed] [Google Scholar]
- 25.Bindhu J., Supe S., Pawar Y. Intensity modulated radiotherapy (IMRT) the white, black and gray: a clinical perspective. Rep Pract Oncol Radiother. 2009;14:95–103. [Google Scholar]
- 26.Joseph B., Supe S.S., Ramachandra A. Cyberknife: a double edged sword? Rep Pract Oncol Radiother. 2010;15:93–97. doi: 10.1016/j.rpor.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Zhao C., Ghimire B. The role of concurrent chemoradiotherapy in the treatment of locoregionally advanced nasopharyngeal carcinoma among endemic population: a meta-analysis of the phase III randomized trials. BMC Cancer. 2010;10:558. doi: 10.1186/1471-2407-10-558. [DOI] [PMC free article] [PubMed] [Google Scholar]


