Abstract
Two clusters of forebrain neurons—one in the posterodorsal preoptic nucleus (PdPN) and one in the lateral part of the posterodorsal medial amygdala (MeApd)—are activated at ejaculation in male rats and gerbils as seen with Fos immunocytochemistry. To understand the functions of these cells and how they respond synchronously, it may be useful to identify their neurotransmitters. Nitric oxide (NO) was of interest because its levels in the preoptic area affect ejaculation, and it could synchronize clustered neurons through paracrine/volume transmission. Thus, we determined whether the ejaculation-related cells produce NO by assessing Fos co-localization with NO synthase (NOS) in recently mated male gerbils. We also studied NOS-Fos co-localization in the medial part of the medial pre-optic nucleus (MPNm), where half of the neurons that express Fos after mating reflect ejaculation. We also quantified NOS co-localization with androgen receptor (AR) and NOS sensitivity to androgens at these sites. Without quantification, we extended these analyses throughout the hypothalamus and amygdala. Many mating-activated PdPN, lateral MeApd, and MPNm cells contained NOS (32–54%), and many NOS neurons at these sites expressed Fos (34–51%) or AR (25–69%). PdPN and MPNm NOS cells were sensitive to testosterone but not its androgenic metabolite dihydrotestosterone. The overall distribution of NOS and NOS-AR cells was similar to that in rats. These data suggest that NO may help to synchronize the activation of PdPN and lateral MeApd neurons at ejaculation and that NOS in PdPN and MPNm cells is regulated by testosterone acting via estradiol or without undergoing metabolism.
INDEXING TERMS: posterodorsal preoptic nucleus, posterodorsal medial amygdala, medial preoptic nucleus, male sex behavior, bed nucleus of the stria terminalis, paracrine neurotransmission
Two distinctive clusters of forebrain neurons—one in the posterodorsal preoptic nucleus (PdPN) and one in the lateral part of the posterodorsal medial amygdala (MeApd)—are activated at ejaculation in male rats and gerbils, as assessed by Fos immunocytochemistry (ICC; Baum and Everitt, 1992; Coolen et al., 1996; Heeb and Yahr, 1996). These clusters are not seen in males that achieve an equal number of intromissions if the male is separated from the female before ejaculation. Thus, during or shortly after the ejaculatory intromission, PdPN and lateral MeApd neurons either receive different inputs than they did with prior intromissions or respond differently to the same inputs.
To understand the functions of the neurons associated with ejaculation, it may be necessary to manipulate them independently of their neighbors. However, because the PdPN and lateral MeApd are small (≈150 μm diameter) and include or are near cells that affect mounting, it is difficult to influence the ejaculation-related cells without producing confounding behavioral deficits (Heeb and Yahr, 2000). Like the PdPN and lateral MeApd, the medial part of the medial preoptic nucleus (MPNm) expresses Fos with mating, but its activated cells are not clustered and only some reflect ejaculation (Baum and Everitt, 1992; Coolen et al., 1996; Heeb and Yahr, 1996). Therefore, identifying the projections (Heeb and Yahr, 2001; Simmons and Yahr, 2002) and neurotransmitters (Simmons and Yahr, 2003) of the mating-activated cells in these brain areas could suggest methods for more selective manipulations to further elucidate their functions.
With this in mind, we asked whether the cells associated with ejaculation in the PdPN and lateral MeApd, and with mating in the MPNm, contain nitric oxide synthase (NOS), the enzyme that catalyzes the production of the signaling molecule, nitric oxide (NO). NO is of interest for two reasons. First, its levels in the preoptic area (POA) are implicated in the control of ejaculation. Blocking NO synthesis in the medial POA (MPOA) during copulation inhibits ejaculation in male rats (Lagoda et al., 2004), whereas applying a NO donor facilitates it (Sato et al., 2007). Second, the ability of NO to move freely across membranes may allow it to synchronize activation of PdPN and lateral MeApd cells. Because NO produced by one cell can enter and affect others, it could coordinate responses of clustered neurons via paracrine/volume transmission (Garthwaite and Boulton, 1995; Fuxe et al., 2007; Steinert et al., 2008). These properties of NO render it a likely candidate as a trigger for ejaculation or its sequelae.
After examining the presence of NOS in mating-activated PdPN, lateral MeApd, and MPNm cells, we investigated whether the NOS-containing cells express androgen receptor (AR) and whether NOS levels within them are regulated by androgens. In gerbils and rats, testosterone (T) promotes mounting by metabolism to estradiol but promotes ejaculation via its androgenic metabolite dihydrotestosterone (DHT; Baum and Vreeburg, 1973; Yahr and Stephens, 1987). Both T and DHT bind to AR, but their relative potencies after binding vary by tissue (Askew et al., 2007). Thus, if PdPN or lateral MeApd cells are sites where T or DHT acts to affect ejaculation, we would expect them to contain AR. Also, if NOS is among the molecules regulated by AR in those cells, it should respond to T and/or DHT. Neurons in the medial amygdala (MeA) enlarge when exposed to T (Cooke et al., 1999), so how T and DHT affect the size and shape of NOS cells in the lateral MeApd was assessed as well. Finally, NOS-AR co-localization and NOS sensitivity to androgens were qualitatively analyzed in the entire hypothalamus, amygdala, and bed nucleus of the stria terminalis (BST) because NOS and NOS-AR distributions vary (Vincent and Kimura, 1992; McDonald et al., 1993; Satoh et al., 1995; Hadeshi and Wood, 1996; Yamada et al., 1996).
MATERIALS AND METHODS
Subjects
All gerbils (Harlan Sprague-Dawley, Indianapolis, IN) were housed in same-sex pairs under a 14:10-hour light/ dark cycle with food and water freely available. The females used as partners in tests of male sex behavior were implanted subcutaneously (sc), 5–10 days before use, with 5-mm Silastic capsules (1.57 mm i.d., 3.16 mm o.d.) filled with estradiol benzoate to induce sexual receptivity. All animals were anesthetized with sodium pentobarbital injected intraperitoneally before surgery (50 mg/ kg) or perfusion (100 mg/kg). All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Behavioral testing
Males gained sexual experience by being paired with receptive females on two to three occasions. Each male was placed in a clear Plexiglas arena (37–42 cm diameter, 51 cm high) with bedding on the floor. A female was introduced 5 minutes later, and the male was given 30 minutes to ejaculate. Only males that ejaculated during at least two tests were used. Males used to study Fos expression were re-exposed to females on the day of perfusion. For those tests, the male was allowed 30 minutes to intromit. If he did not do so within 15 minutes, a different female was used for the rest of the test. If he intromitted, he was given 30 minutes to ejaculate. If he ejaculated, he was returned to his home cage and perfused 1–1.5 hours later.
Hormone manipulations
Males used to study androgenic effects on NOS staining as assessed by nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d) histochemistry were randomly assigned to four groups of four. One group was sham-operated and implanted sc with empty, 5-mm, Silastic capsules. The others were castrated and given empty capsules or capsules filled with T or DHT. T capsules this size produce physiological T levels (Clark et al., 1993) and maintain mating in castrated males (Yahr et al., 1979). Eight weeks later, males were perfused for histochemistry. At perfusion, seminal vesicles were removed and stored in Bouin’s solution (Sigma, St. Louis MO) until they were cleaned and weighed. Their weights conformed to previous results (Yahr and Stephens, 1987), confirming androgen release.
Tissue fixation and sectioning
Males were perfused with saline (0.9%) followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB; pH 7.4; 150 ml over 10 minutes). Brains were postfixed for 2 hours in cold (4°C) fixative, stored overnight in cold PB with 20% sucrose, frozen, and cut coronally at 30 μm. Every section through the POA and MeA was collected in cold PB. For co-localization, sections from five males were collected as two sets of alternates. One set was processed for Fos ICC and the other for AR ICC. In tissue from two other males, NOS and AR were visualized in every section. For two males, sections were also collected through the midbrain retrorubral field, but these sections were processed for histochemistry only.
NADPH-d histochemistry
Visualization of NOS by NADPH-d histochemistry preceded ICC for AR or Fos. Because the only NADPH-d activity detected in forebrain neurons after paraformaldehyde fixation reflects the catalytic activity of NOS (Dawson et al., 1991; Hope et al., 1991), we refer to cells visualized here as NOS cells. In paraformaldehyde-fixed brain tissue, both constitutive forms of NOS, i.e., neuronal NOS (nNOS) and endothelial NOS (eNOS), can display NADPH-d activity (Gabbot and Bacon, 1993; Tomimoto et al., 1994). Moreover, some neurons use eNOS to make NO. However, NADPH-d activity of eNOS in neurons is seen only after glutaraldehyde fixation, at least in the hippocampus (Dinerman et al., 1994). Thus, we were more likely to visualize NOS cells containing nNOS than eNOS, and, in tissue from four males, two of which had been castrated, that we stained for nNOS by ICC, the distribution of cells seen was the same as with NADPH-d histo-chemistry. Nonetheless, for our purposes, i.e., studying neurons that make NO, it did not matter whether they make NO with nNOS or eNOS.
Sections were rinsed twice, with agitation, over 5 minutes in cold PB (pH 7.4) and incubated in the dark, without agitation, for 1–1.5 hours at 37°C in PB containing 0.3% Triton X-100, 0.5 mg/ml nitroblue tetrazolium chloride (NBT; Sigma), and 1.0 mg/ml β-NADPH-d (Sigma). The reaction, which produces a blue product, was monitored microscopically and stopped by adding purified water when PdPN and lateral MeApd cells were distinct (usually 3–4 hours). By this time, cells in many other areas were dark blue. Sections were then rinsed once for 5 minutes with purified water and twice over 20 minutes with PB. Tissue from several males was processed at a time to reduce variability. Tissue from hormonally manipulated males was processed in four runs (1 male/group/run), and slides were coded to prevent knowledge of the treatment group or run.
Antibody characterization
The antibodies and antiserum used are listed in Table 1. The rabbit polyclonal antibody against AR was characterized for rat prostate by Prins et al. (1991). On Western blots of protein extracts from cultured cerebellar granule cells of neonatal rats, it reacted with a single band at 110 kDa (Ahlbom et al., 2001). On Western blots of proteins extracted from the MPOA of adult male and female mice, it reacted with a single band at 97 kDa (Lu et al., 1998). In our hands, preabsorbing the antibody with the antigen abolished immunoreactivity, but preabsorbing it with a peptide corresponding to amino acids 462–478 of rat AR did not. Both of those peptides were also provided by Dr. Prins. The distribution of staining obtained with this antibody matched that seen with autoradiography using 3H-DHT (Commins and Yahr, 1985).
TABLE 1.
Primary Antibodies
| Antigen | Immunogen | Source | Dilution |
|---|---|---|---|
| Androgen receptor (AR) | Rat AR amino acids 1–21 | G. Prins (University of Illinois, Chicago, IL) | 1:20,000 |
| c-Fos | Synthetic SGFNADYEASSSRC peptide, which corresponds to human c-Fos residues 4–17 | Oncogene (San Diego, CA); Ab-5; cat. no. PC38 | 1:50,000 |
| Neuronal NOS (nNOS) | Recombinant human nNOS | Chemicon (Temecula, CA); AB5380, lot 21011021 | 1:7,000 |
The rabbit polyclonal antiserum against c-Fos recognizes the ~55-kDa c-Fos protein as well as v-Fos (they share the immunogen sequence) but does not recognize the ~39-kDa Jun protein, per information provided by the vendor. Preabsorbing the serum with Fos protein (Elmquist et al., 1996) or the synthetic peptide immunogen (Serrats and Sawchenko, 2006) eliminated staining in rat brain. In rat brain, this antiserum also co-localized with c-fos mRNA under a variety of conditions (Serrats and Sawchenko, 2006). In our hands, it produced the same pattern of neuronal labeling in the brains of recently mated male gerbils that we saw (Heeb and Yahr, 1996) using a c-Fos antibody from a different source that was made against amino acids 3–16 at the amino terminus of human c-Fos and which has been shown by Western blot analysis to detect a specific p62 band for which staining was eliminated by exposure to a 10-fold excess of the specific immunizing peptide (Preston et al., 1996). The staining pattern seen in gerbils with these c-Fos antibodies also matches that seen in recently mated male rats (Baum and Everitt, 1992; Coolen et al., 1996).
The rabbit polyclonal antibody against nNOS reacts, per the vendor’s information sheet, with nNOS from rats and mice but does not cross-react with endothelial or inducible NOS. Preincubating the antibody with the immunizing protein eliminated staining in the lateral geniculate nucleus of rats (Hwang and Huh, 2010). On Western blots of reduced, denatured proteins isolated from lysed, fetal-rat cortical neurons grown in primary culture and exposed to various steroid treatments, the antibody produced a single band of 140 kDa (Mannella et al., 2009). In our hands, it produced neuronal staining corresponding to that seen with NADPH-d histochemistry as illustrated below.
Fos, AR, and nNOS ICC
Unless noted, incubations and rinses were done at room temperature with gentle agitation, and sections were rinsed in PB with saline (PBS; pH 7.4, 3X over 15 minutes) after each incubation. Sections were incubated 1–1.5 hours in 10% normal goat serum (NGS) plus 0.3% Triton X-100 in PBS. After that solution was removed (no rinse), sections were incubated for 48–72 hours (18–24 hours for nNOS ICC) at 4°C in carrying solution (0.3% Triton X-100 + 5% NGS in PBS) containing the primary antibody; 1–1.5 hours in carrying solution containing biotinylated goat anti-rabbit IgG at 1:200 (Vector, Burlingame, CA); 1–1.5 hours in Vectastain Elite ABC (Vector) solution; and in 0.01% H2O2 plus 0.05% 3,3′-diaminobenzidine in PBS. The last reaction, which gives a brown product, was monitored microscopically and stopped by adding PBS, usually after 5–10 minutes. Sections were mounted, air-dried, dehydrated in alcohols, delipidized very briefly in xylene, and coverslipped by using Permount (Fisher Scientific, Pittsburgh, PA).
Sections processed for NADPH-d histochemistry with either the β-NADPH-d or NBT omitted, or for nNOS ICC without the primary antibody, showed no staining. When sections processed for NADPH-d activity were not processed further, or were processed for Fos or AR ICC without primary antibody, no brown reaction product was seen.
Histological analysis and imaging
Distributions of NOS (NADPH-d histostained) and NOS-AR cells were studied from the rostral POA to the ventral premammillary nucleus of the hypothalamus and throughout the amygdala and BST. Cell groups were identified with rat brain maps and atlases (Swanson, 1992; Paxinos and Watson, 1997) and descriptions of the gerbil POA (Commins and Yahr, 1984). We previously referred to some cell groups studied here as parts of the sexually dimorphic preoptic area (SDA) of the gerbil hypothalamus (Commins and Yahr, 1984), but based on homologies discussed in Finn et al. (1993), we now refer to them as parts of the medial preoptic nucleus (MPN). Thus, the medial SDA, the area lateral to it and the SDA pars compacta, are now referred to as the MPNm, lateral MPN (MPNl), and central MPN (MPNc), respectively. Based on similarities between the lateral SDA and parts of the rat BST as discussed in Finn and Yahr (2005), we now refer to the lateral SDA as the ventral BST (Finn and Yahr, 2005). It corresponds primarily to the magnocellular sub-nucleus of the BST and the parts of the ventral subnucleus lying ventral and lateral to the magnocellular BST (Ju and Swanson, 1989).
NOS-Fos and NOS-AR co-localization were quantified in the MPNm, PdPN, and lateral MeApd using 4–5 males/ antigen/site. At 312× (PdPN, MPNm) or 500× (lateral MeApd), cells in boxes superimposed on the tissue with a drawing tube were counted bilaterally in nonadjacent sections (1–2/antigen/side). The PdPN box (0.13 × 0.13 mm tissue) was placed over the cluster of immunoreactive (IR) cells. For sections not processed for Fos, it was positioned so that its lateral edge was just lateral to the fornix and its top edge was just below the top of the third ventricle (below the fornix). This was done at the most caudal level of the anterior commissure (ac) or just caudal to it. The lateral MeApd box (0.16 × 0.16 mm) was placed over the Fos-IR cell cluster or just inside the MeApd, where its lateral edge, as revealed by the stria terminalis, was furthest from the optic tract and most concave. This was done 30–150 μm rostral to the lateral ventricle. The MPNm box (0.12 × 0.24 mm) was oriented vertically within the MPNm at the level of the MPNc with its top lateral corner ventromedial to the MPNc, which usually stood out with NOS staining.
Methods for positioning the boxes that defined the counting areas over the PdPN and lateral MeApd were developed using tissue taken from males that had copulated to ejaculation and that was processed for Fos ICC. This method allowed us to position the counting area over the sites of the ejaculation-related Fos clusters when visualization of Fos was not possible, such as in conjunction with ICC for AR, or when males would not be able to copulate to ejaculation, such as after castration. Testing on sections from other males processed for Fos ICC after ejaculation showed that the protocol used here reliably positioned counting areas so that they largely or completely overlapped the ejaculation-related clusters in two to three sections. Positioning the MPNm counting area in relation to, but not overlapping, the MPNc was done to make the area in which MPNm cells were counted as consistent and homogeneous as possible.
Fos- or AR-IR nuclei in or near the box, including those in double-labeled cells, were drawn while the tissue was viewed with a blue glass filter. They were identified as round, brown profiles of a size consistent with neuronal cell nuclei. Then, using a yellow filter (Wratten #15; Kodak, New York, NY), NOS cells were drawn, including double-labeled cells. They were identified by outlines, consistent with neuronal soma, that were distinct from the surround due to dark blue cytoplasmic reaction product. Finally, double-labeled cells were specifically identified.
From the drawings, NOS cells and IR nuclei inside the box or overlapping its top or right edges were counted. NOS cells overlapping the left or bottom edges were counted if at least half of the soma was in the box. IR nuclei overlapping those edges were counted if at least half of the nucleus was in the box or if the cell was double-labeled and at least half of its soma was in the box. Then, double-labeled cells were counted. For each male, the percentage of Fos- or AR-IR cells containing NOS was calculated by dividing the number of double-labeled cells by the number of Fos- or AR-IR nuclei, respectively. Similarly for each male, the percentage of NOS cells that were Fos- or AR-IR was calculated by dividing the number of cells double-labeled for Fos and NOS, or AR and NOS, respectively, by the number of NOS cells.
Tissue from hormonally manipulated males was analyzed the same way using one section per side. For the PdPN and lateral MeApd, the sections chosen per side were those with the greatest density of NOS cells in the prescribed counting area. MPOA tissue from one T-treated male was not usable, leaving three in that group for the PdPN and MPNm. The intensity of NOS fiber staining in the MPNc relative to the surround was also noted in all four groups, and NOS cell density in the principal sub-nucleus of the BST (BSTpr) just below the lateral ventricle was recorded as low, moderate, or high. To assess the effects of androgens on NOS cell size and shape in the lateral MeApd, cell bodies (10/side/male) with pale nuclei surrounded by blue cytoplasm were traced at 500× for one section/side, and scanned into Sigma Scan Pro (Jandel Scientific, San Raphael, CA), which computed their areas and circularity. Mean values for each side were calculated for each male. NOS staining in castrated vs. gonadally intact males was noted in other areas but not quantified.
Digitized images were acquired with a Zeiss AxioImager M2 light microscope using a 10×, 20× or 40× oil objective and AxioVision software v4.7. To reproduce the appearance of the tissue at microscopy, images were adjusted for tonal and/or color qualities by using Adobe (San Jose, CA) Photoshop CS3 (levels, curves, and color balance tools). Figures were then assembled in Adobe Illustrator CS3.
Statistics
NOS cell counts for hormonally manipulated males were compared by analysis of variance using a 4(hormone treatment) × 4(histochemical run) design followed by orthogonal contrasts of males with versus without T, males with endogenous versus exogenous T, and castrates given DHT versus vehicle. Although not independent of those comparisons, selected contrasts were also done for the MPNm comparing T- and DHT-treated castrates with each other and comparing T-treated castrates with castrates with empty implants. Statistical analyses were done with SYSTAT (Chicago, IL) v.8 software. Because it flagged a male in the DHT-treated group as an extreme outlier for PdPN and lateral MeApd cell counts (Studentized residual = 3.77–6.10), analyses for each region were repeated without him. The data presented are from the second analysis, but data for the excluded male are given in the relevant figure caption. Ratings of NOS staining in the MPNc and of NOS cell density in the caudal BSTpr were analyzed by Fisher exact probability and Wilcoxon sign tests, respectively. After data on the size and circularity of lateral MeApd somas were compared for the left and right sides with t-tests for correlated means, the data for the two sides were summed and group differences assessed as above. For four males used to co-localize NOS with both Fos and AR, the number of NOS cells seen in the PdPN and the total seen across all areas studied for both types of co-localization in those males were compared with t-tests for correlated means to determine whether the type of co-processing affected NOS cell counts.
RESULTS
NOS-Fos co-localization after mating
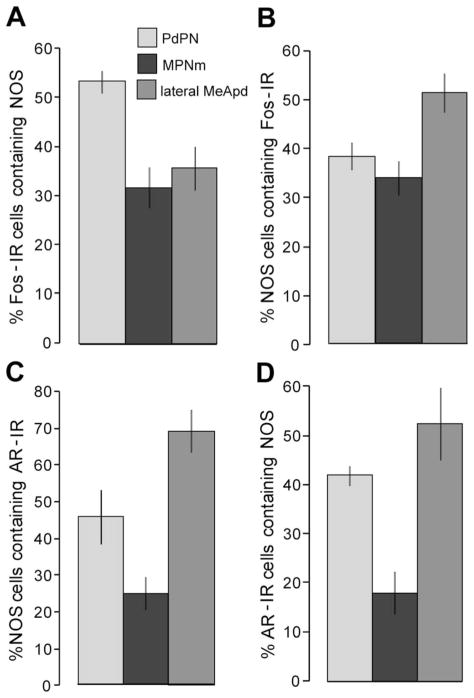
As shown in Figure 1A, half (54%) of the PdPN cells that were Fos-IR after mating contained NOS. About a third of Fos-IR cells contained NOS in the lateral MeApd (36%) and MPNm (32%). Fos was seen in 38% of NOS cells in the PdPN, 51% in the lateral MeApd, and 34% in the MPNm (Fig. 1B). The numbers of cells containing NOS, Fos, or both are given in Table 2. The number expressing Fos was similar to what was seen previously in tissue processed for co-localization with other molecules (Simmons and Yahr, 2002, 2003). These data are illustrated in Figure 2A, B, D, E, G, H.
Figure 1.
Percentages of cells in the PdPN, MPNm, and lateral MeApd of male gerbils in which NOS was co-localized with AR or with Fos after ejaculation. A: Percent of Fos-IR cells in recently mated males that contained NOS. B–C: Percent of NOS cells that expressed Fos after mating (B) or that contained AR (C). Percent of and AR-IR cells that contained NOS (D). The areas in which cells were counted measured 0.0169, 0.0288, and 0.0256 mm2 for the PdPN, MPNm, and lateral MeApd, respectively. Note that the y-axis in C differs from that in A, B, and D. Data are shown as group means ± SEM; n = 4–5 per group. For abbreviations, see list.
TABLE 2.
Number of PdPN, Lateral MeApd, and MPNm Cells1 Positive (+) for NOS Based on NADPH-d Activity, Immunoreactive (IR) for Mating-Induced Fos or AR, or Double-Labeled for Both
| Co-localization of NOS and Fos
|
Colocalization of NOS and AR
|
|||||
|---|---|---|---|---|---|---|
| NOS+ | Fos-IR | Both | NOS+ | AR-IR | Both | |
| PdPN2 | 49 ± 4 | 35 ± 3 | 19 ± 2 | 67 ± 10 | 69 ± 10 | 29 ± 4 |
| Lateral MeApd | 48 ± 7 | 68 ± 4 | 25 ± 4 | 75 ± 13 | 98 ± 11 | 54 ± 14 |
| MPNm2 | 40 ± 3 | 42 ± 2 | 13 ± 2 | 51 ± 6 | 73 ± 10 | 13 ± 3 |
Per 0.0169 (PdPN), 0.0256 (lateral MeApd), and 0.0288 (MPNm) mm2. For abbreviations, see list.
n = 5 for NOS-Fos co-localization; n = 4 in all other cases.
Figure 2.
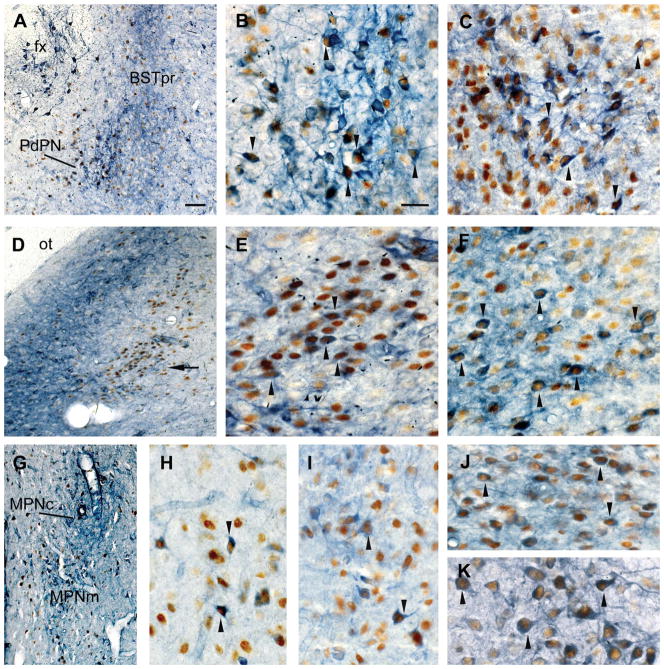
NOS-Fos and NOS-AR co-localization in male gerbils. Photomicrographs of coronal sections showing the co-localization of NOS (blue-purple) and Fos after ejaculation (brown; A, B, D, E, G, H), or the co-localization of NOS and AR (brown; C, F, I–K), in and near the PdPN (A–C), lateral MeApd (D–F), MPNm (G–I), BSTpr (J), and VMH (K). A: Fos-IR cells are intermingled with NOS cells in the PdPN and BSTpr of recently mated males. The BSTpr contains a dense plexus of NOS fibers, and a distinct circle of NOS cells can be seen just below the fornix (fx). B: Higher magnification of the PdPN shown in A showing NOS-Fos double-labeled cells (arrowheads). C: NOS-AR double-labeled cells are common in the PdPN (arrowheads). D: Dense plexus of NOS fibers surrounding NOS cells in the medial MeApd, near the optic tract (ot), but not NOS cells in the lateral MeApd, where a cluster of Fos-IR cells appears at ejaculation (arrow). E: Higher magnification of the lateral MeApd Fos cluster in D showing NOS-Fos double-labeled cells (arrowheads). F: NOS-AR double-labeled cells (arrowheads) are abundant in the lateral MeApd. G: NOS and Fos-IR cells in the MPNm. The MPNc has few, if any, Fos-IR cells but is filled with NOS fibers. H: Higher magnification of the MPNm in D showing NOS-Fos double-labeled cells (arrowheads). I: Some NOS-AR double-labeled cells (arrowheads) were found in the MPNm. J, K: The dorsal BST (J) and VMH (K) had many NOS-AR double-labeled cells (arrowheads). Arrowheads point to only some of the double-labeled cells visible in B, C, E, F, H–K. For abbreviations, see list. Scale bar = 50 μm in A (also applies to D, G; 10× objective); 20 μm in B (also applies to C, E, F, H–K; 40× oil objective).
NOS cell distribution and co-localization with AR
NOS cells were moderately dense in the MPNm, the ventral BST, and the bridge of cells that connects them in gerbils. NOS cells were moderately dense in the MPNl as well. Two parts of the MPN had distinctive NOS histo-staining: the MPNc, which was filled with NOS fibers (Fig. 2G), and the caudodorsomedial corner of the MPNm (Ulibarri and Yahr, 1993, 1996), which was filled with dark NOS cells. AR was seen in some NOS cells in the ventral BST and in each part of the MPN. In the MPNm, 25% of NOS cells contained AR (Figs. 1C, 2I), and 18% of cells with AR contained NOS (Fig. 1D, Table 2).
AR was also seen in some NOS cells in surrounding areas. Rostrally, these included the median and anterodorsal preoptic nuclei, where NOS cells were dense and dark, and a diagonal band of dark cells that extended from the anteroventral preoptic nucleus into the lateral POA. NOS cells were lighter and sparser lateral to the anteroventral periventricular nucleus (AVPv), and even sparser within it, but some had AR. Caudally, the nucleus of the lateral olfactory tract and the diagonal band of Broca had many NOS cells, whereas the suprachiasmatic nucleus had few, but each of these three areas had some NOS cells with AR.
The PdPN, particularly its caudal part, contained dark NOS cells, as shown in Figure 2A–C. Among PdPN NOS cells, 46% had AR (Figs. 1C, 2C, Table 2), and among PdPN cells with AR, 42% contained NOS (Fig. 1D, Table 2). Dark NOS cells were seen throughout the BSTpr but became denser caudally, particularly just below the lateral ventricle, as shown in Figure 3A. Many NOS cells there had AR (Fig. 2J). Dark NOS cells were also clustered amid fibers of the stria terminalis lateral to the anterodorsal BST and in a circle below the fornix (fx) and bed nucleus of the ac (Fig. 2A), but almost none of them had AR. The ventral and anterodorsal BST had fewer and lighter NOS cells, and some in the anterodorsal BST had AR.
Figure 3.
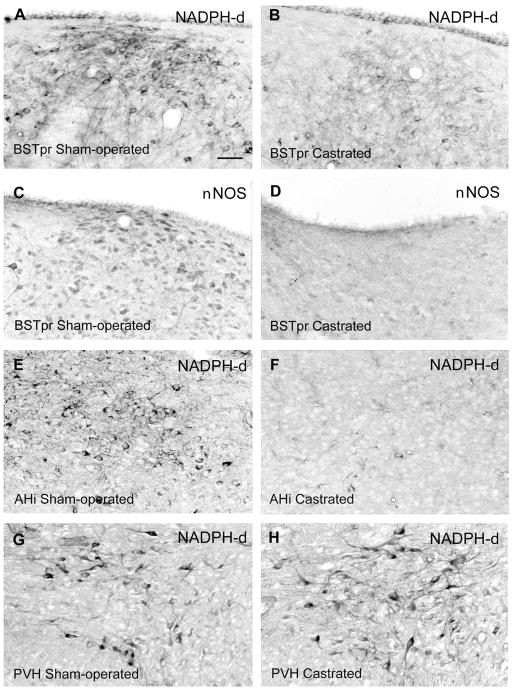
Distribution of NOS cells in the gerbil BSTpr (A–D), AHi (E, F), and PVH (G, H) and effects of castration on NOS cells in the BSTpr and AHi but not the PVH. Photomicrographs show NOS cells in males that were sham-operated (A, C, E, G) vs. castrated (B, D, F, H). All of the males used for NADPH-d histostaining were given empty Silastic implants, and each pair of sections is from males processed in the same NADPH-d staining set. The two males used for E and F were also used for G and H. C and D show the effect of castration in the dorsal BSTpr as visualized by ICC for nNOS for comparison with the effects seen with NADPH-d histostaining. For abbreviations, see list. Scale bar = 40 μm in A (applies to A–H).
The anterior and dorsomedial hypothalamic nuclei and the anterior parvicellular part of the paraventricular nucleus of the hypothalamus (PVH; Fig. 3G) had moderate numbers of NOS cells, with their density increasing caudally in the parvicellular PVH, but NOS cells in these areas seldom had AR. NOS cells were sparse in the arcuate and retrochiasmatic nuclei, but a thick band of dark cells in the lateral hypothalamic area extended ventrally into the supraoptic nucleus, where many NOS cells had AR. AR was also common in the NOS cells in the ventral premammillary nucleus and the lateral part of the ventro-medial nucleus of the hypothalamus (Fig. 2K), both of which also contained a dense plexus of NOS fibers.
The MeA had more NOS histostaining than any other area examined and had some of the highest levels of NOS-AR co-localization. Dark NOS cells and fibers filled the anterodorsal MeA and continued into the caudal and medial MeApd, as shown in Figure 2D, which contrasted with the light fiber staining and moderate number of NOS cells in the lateral MeApd (Fig. 2D). However, many NOS cells in each of these areas had AR. In the lateral MeApd, 69% of NOS cells contained AR (Figs. 1C, 2F), and 53% of AR cells contained NOS (Fig. 1D, Table 2). NOS cells were seldom seen in the posteromedial division of the cortical nucleus of the amygdala but were common in its anterior and postero-lateral regions, where many had AR. NOS cell density was low in the central, lateral, and basolateral nuclei of the amygdala, moderate in the posteroventral MeA, and high in the anterior amygdaloid nucleus, the anterior part of the basomedial amygdala, and the amygdalohippocampal area (AHi; Fig. 3E). Few NOS-AR cells were seen in any of these areas except the AHi, where they were moderately dense.
Many NOS cells were seen in the peripeduncular nucleus of the midbrain, and some were scattered in the retrorubral field and periaqueductal gray. These sections were not stained for AR.
Table 2 shows the number of PdPN, MPNm, and lateral MeApd cells containing NOS, AR, or both. It suggests that the number of NOS cells seen with NADPH-d histochemistry may have varied depending on whether it was followed by AR or Fos ICC; however, this difference involves both within- and between-subject effects. Although four to five males were used to quantify co-localization at each site, the same males could not be used to quantify each antigen at each site. For the PdPN, both types of co-localization were assessed in four males. Two of them were also used to quantify both types of co-localization at a second site, and one was used for both at all three sites. In these males, the number of PdPN NOS cells (mean ± SEM) seen after Fos (97 ± 10) versus AR (135 ± 19) ICC did not differ [t(3) = 2.09, P = 0.13], nor did the total number of NOS cells seen across all regions for which they were used for both types of co-localization [194 ± 43 vs. 195 ± 29, respectively; t(3) = 0.02, P = 0.99].
Effects of androgens on NOS cells and fibers
The effects of castration on histochemical visualization of NOS cells in the PdPN, MPNm, and lateral MeApd of male gerbils are shown in Figures 4, 5 and 6. Cell counts in these areas showed that males exposed to T (endogenous or exogenous) had more NOS cells at each site than castrates given DHT or vehicle, as summarized in Figure 7 [PdPN:F(1,7) = 51.77, P < 0.001; overall F(3,7) = 18.36, P < 0.002; MPNm:F(1,7) = 11.98, P < 0.02; overall F(3,7) = 6.87, P < 0.02; lateral MeApd: F(1,8) = 6.92, P = 0.03; overall F(3,8) = 2.46, P = 0.14]. No significant differences were noted in any of these areas when T-treated castrates were compared with sham-operated males, or when DHT-treated castrates were compared with castrates given empty capsules. In the MPNm, T-treated castrates did not differ from castrates given DHT; however, unlike the castrates given DHT, they did differ from castrates with empty implants [F(1,7) = 9.88, P = 0.016]. Differences across histochemical runs (or time between tissue processing and analysis), which were independent of subject treatment, were also significant for the PdPN [F(3,7) = 15.05, P < 0.003] and nearly so for the MPNm [F(3,7) = 4.18, P = 0.054] but not for the lateral MeApd. No differences were detected in the lateral MeApd in regard to NOS cell size or shape (circularity) as a function of hemisphere, histochemical run, or hormone treatment.
Figure 4.
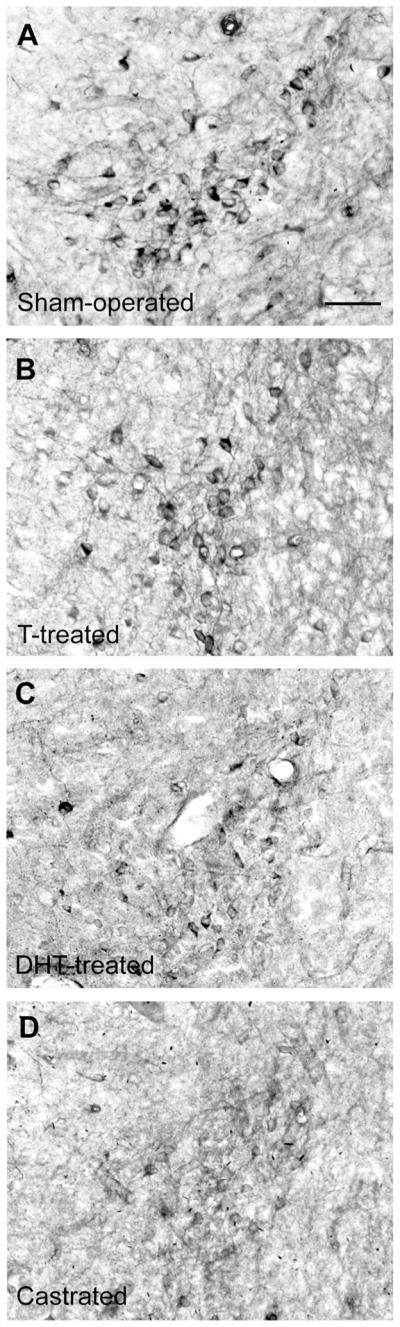
Effects of castration and androgen replacement on NOS cells in the gerbil PdPN. Males were sham-operated and given empty implants (A) or castrated and implanted sc with Silastic capsules containing T (B), DHT (C), or no hormone (D). Photomicrographs show males from the same NADPH-d staining set. For abbreviations, see list. Scale bar = 40 μm in A (applies to A–D).
Figure 5.
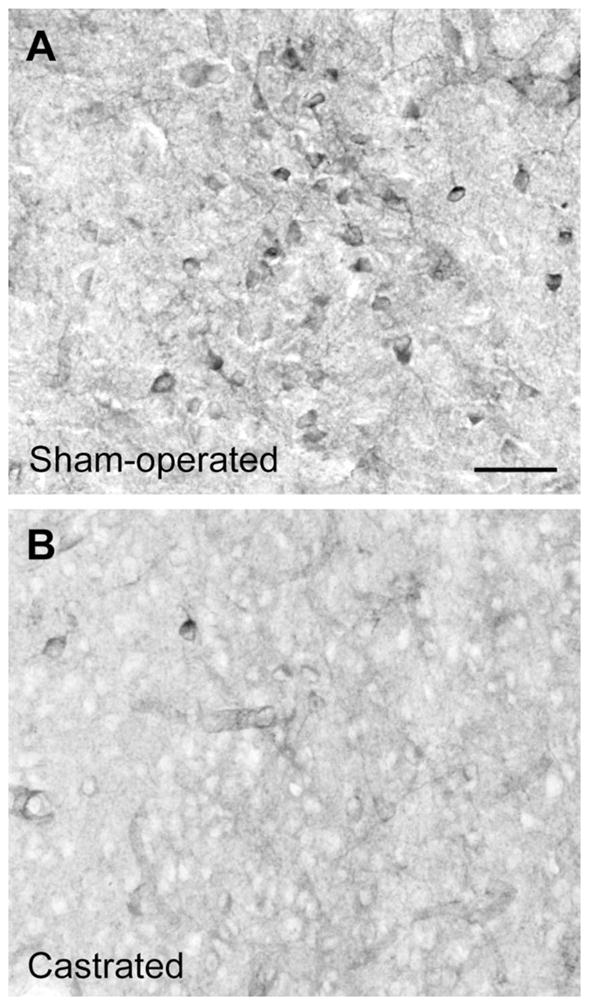
Effect of castration on NOS cells in the gerbil MPNm. Photomicrographs show NOS cells in the MPNm of males that were sham-operated (A) or castrated (B), given empty implants, and processed in the same NADPH-d staining set. Scale bar = 40 μm in A (applies to A, B).
Figure 6.
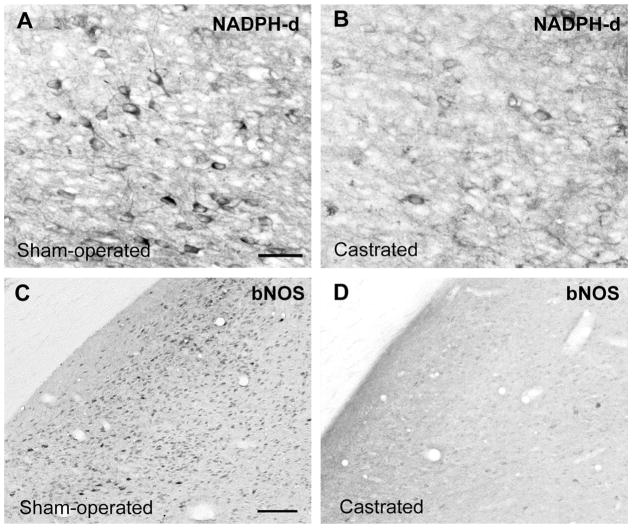
Effect of castration on NOS cells in the gerbil lateral MeApd. Photomicrographs show NOS cells in the lateral MeApd of males that were sham-operated (A, C) or castrated (B, D). The cells were visualized by NADPH-d staining (A, B) or nNOS ICC (C, D). Both males used for NADPH-d staining were given empty Silastic implants and were processed in the same staining set. For abbreviations, see list. Scale bar = 40 μm in A (applies to A, B) and C (applies to C, D). lesions.
Figure 7.

Effects of castration and androgen replacement on NOS cell counts in the PdPN, MPNm, and lateral MeApd of male gerbils. Areas in which cells were counted are the same as in Figure 1. Data are shown as group means ± SEM; n = 3–4 per group. A DHT-treated male flagged as an outlier for cell counts in the PdPN and lateral MeApd (Studentized residual = 3.77–6.10) was excluded from the analyses and this graph. His counts for the PdPN, MPNm, and lateral MeApd were 46.0, 26.50, and 57.5, respectively. For abbreviations, see list.
NOS histostaining in the MPNc and caudal BSTpr also responded to T but not DHT. In the caudal BSTpr, males exposed to endogenous or exogenous T scored higher on NOS cell density than castrates given DHT or vehicle (Wilcoxon Z = 2.42, P < 0.03), as shown in Figure 3A vs. B. In the MPNc, NOS fiber staining showed the same pattern (Fig. 8). In males in which the MPNc could be clearly identified, NOS fibers were darker than in the surround in the six exposed to T but were lighter than in the surround in the seven lacking T (Fisher exact P = 0.001).
Figure 8.
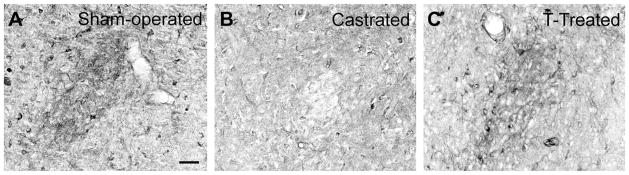
Effects of castration and T replacement on NOS fibers in the gerbil MPNc. Photomicrographs of NOS fibers in the MPNc of a sham-operated male given an empty Silastic implant (A), a castrate given an empty implant (B), and a castrate given an implant containing T (C). All three males were from the same NADPH-d staining set. Scale bar = 50 μm in A (applies to A–C).
Relative to sham-operated controls, castrated controls appeared to have fewer NOS cells in the posteroventral MeA, AHi, and parts of the MeApd other than the lateral MeApd. This was most pronounced in the caudal MeApd and AHi (Fig. 3E vs. F). In contrast, castration had no apparent effect on NOS cell numbers or overall histo-staining in the anterodorsal MeA, anterior amygdaloid nucleus, anterior basomedial nucleus of the amygdala, lateral BST, or telencephalic sites outside the amygdala or BST, such as the nuclei of the lateral olfactory tract or the diagonal band of Broca. Similarly, castration did not appear to affect NOS staining in the MPNl, the cell group in the caudodorsomedial corner of the MPNm, the AVPv, the median and anterodorsal preoptic nuclei, or the PVH (Fig. 3G vs. H).
We made no attempt to quantify the vasculature but did not note any relationship between vascular staining and the experimental manipulations.
DISCUSSION
Using NADPH-d histochemistry to visualize NOS neurons in the brain, the present study showed that many mating-activated cells in the gerbil PdPN, lateral MeApd, and MPNm contain NOS, and that many NOS neurons at these sites express Fos. Furthermore, NOS-containing cells in all three brain areas express AR and respond to T. DHT did not affect the number of NOS-containing cells in any area studied. Thus, NOergic transmission is likely to play an important role in the PdPN, MPNm, and lateral MeApd during mating and is a target for T, acting via estradiol or direct binding to AR, in all three sites.
Functions of NOS neurons activated with mating
Among MPNm cells activated with mating, 32% contained NOS. That is too few to conclude that any of them are related to ejaculation but high enough to conclude that some are related to mating. In the gerbil MPNm, 90% of Fos-IR cells seen after mating reflect responses to stimuli other than handling, but that percentage is split equally between cells activated at ejaculation and cells responding to cues in the test arena (Heeb and Yahr, 1996). Involvement of MPNm cells in both ejaculation and mating initiation is shown by the effects of cell-body lesions. Asymmetric lesions that disconnect the MPNm only from the MeApd/AHi disrupt ejaculation (Sayag et al., 1994), but bilateral MPNm lesions eliminate mounting (Yahr and Gregory, 1993). In rats, NO levels in the MPOA affect both aspects of mating. Blocking NO synthesis in the rat MPOA blocks the local dopamine release elicited by females and disrupts mounting in sexually naïve males (Lorrain et al., 1996; Lagoda et al., 2004). In sexually experienced males, blocking NO production also disrupts mounting (Sato et al., 1998) but has greater effects on intromission and ejaculation (Lagoda et al., 2004). Applying a NO donor to the MPOA promotes mating, including ejaculation, in castrated males given systemic DHT (Sato et al., 2007), and sexual experience increases the number of NOS cells seen in the MPOA (Dominguez et al., 2006). Thus, the MPNm cells seen here that contained both NOS and Fos after mating could be related to sexual arousal, mating initiation, or ejaculation.
MPNm, or PdPN, cells in which NOS and Fos are co-localized after mating could also be involved in sequelae to ejaculation, such as changes in the secretion of oxytocin (Todd and Lightman, 1986; Carmichael et al., 1987; Hughes et al., 1987; Murphy et al., 1987) or luteinizing hormone (LH; Kamel et al., 1975; Coquelin and Bronson, 1980). NO affects the secretion of LH (Moretto et al., 1993; Rettori et al., 1993), LH-releasing hormone (Bhat et al., 1995; Pu et al., 1996), and oxytocin (Ota et al., 1993; Kadowaki et al., 1994; Kadekaro et al., 1998) in male rats. Even some circulating oxytocin could be produced in the PdPN. Oxytocin has been seen in cells in the rostral part of the anterior commissural nucleus (AC; Paxinos and Watson, 1986; Vanhatalo and Soinila, 1995; Yamada et al., 1996), which is now thought to be the caudal PdPN (Ju and Swanson, 1989; Paxinos and Watson, 1997). Some of those PdPN/ AC cells project to the posterior pituitary, and 70% of those contain NOS (Vanhatalo and Soinila, 1995).
In the PdPN and lateral MeApd, the percentages of cells activated with mating that also contain NOS (54% and 36%, respectively) are high enough to conclude that some of them are related to ejaculation because those percentages are well above those associated with prior events, including intromissions (25% and 14%, respectively; Heeb and Yahr, 1996). If 25% of the Fos-IR PdPN cells seen here were unrelated to ejaculation, and if all of those cells contained NOS, the remaining NOS-Fos cells would still involve 39% of the PdPN cells activated at ejaculation. If none of the cells that expressed Fos in the absence of ejaculation contained NOS, then almost three quarters (72%) of the ones activated at ejaculation produce NO. Using 14% to estimate the percent of Fos-IR cells in the lateral MeApd that are not related to ejaculation, anywhere from 26% to 42% of the ones that are related to ejaculation produce NO.
Cells that produce NO often use it as an intracellular signal, for example, in the pathway leading to Fos production (Haby et al., 1994; Chikada et al., 2000; Gudi et al., 2000). However, because NO freely crosses membranes, NO-producing neurons can affect each other, and cells that do not make NO, via paracrine or volume transmission (Garthwaite and Boulton, 1995; Fuxe et al., 2007; Steinert et al., 2008). NO diffusing out of one cell can enter others to initiate or augment NO-sensitive processes. Such paracrine or volumetric actions could contribute to synchronized activation of neurons, particularly clustered neurons.
Like MPNm cells, PdPN and MeApd cells affect mating. Cell-body lesions at either site disrupt mounting in male gerbils, but in lesioned males that intromit, ejaculation is delayed or eliminated (Heeb and Yahr, 2000). The effects of the PdPN and MeApd on mating may involve the PVH and its effects on erection (Liu et al., 1997). Both the PdPN and MeApd project to the PVH in gerbils (Finn et al., 1993; Simmons and Yahr, 2002). It is not known whether those projections involve cells activated with mating, but in rats, the MeApd-PVH projection arises, in part, from NOS cells (Tanaka et al., 1997). NO levels in the rat PVH rise at erection and stay high throughout copulation (Melis et al., 1998), and disrupting NO production there prevents the reflexive erections that male rats display when near receptive females they cannot touch (Sato et al., 1999). The rat PVH also has cells containing the penile form of nNOS, which is co-localized there with oxytocin (Ferrini et al., 2003).
Among gerbil PdPN cells that express Fos after mating, 43% project to the AVPv, 30% to the dorsal BSTpr, 15% to the MPNm, 12% to the retrorubral field, and 2% to the lateral MeApd (Heeb and Yahr, 2001; Simmons and Yahr, 2002). Unless several of those projections arise from the same cells, it is unlikely that all of the activated PdPN cells containing NOS (54%) are interneurons, and it is likely that NOS cells contribute to at least two activated projections. Among lateral MeApd cells that express Fos after mating, 43% project to the dorsal BSTpr, 27% to the MPNm, 20% to the PdPN, and 18% to the AVPv (Heeb and Yahr, 2001; Simmons and Yahr, 2002). Because NOS is in fewer activated cells here (36%) than in the PdPN, the possibility that all of the double-labeled cells are interneurons is greater. It seems just as likely, though, that some are projection neurons and contribute to more than one activated projection, or that they could all project to the BSTpr.
In regard to neurotransmitter phenotypes, mating-activated PdPN or lateral MeApd cells that make NO may also make a classical transmitter. γ-Aminobutyric acid (GABA) is made in 60% of PdPN cells that are Fos-IR after mating (Simmons and Yahr, 2003), so some or all of the Fos-IR PdPN cells that make NO (54%) could be GABAergic. If those subsets do not overlap, NOergic and GABAergic cells could account for all of the PdPN cells activated with mating. In the lateral MeApd, 45% of cells expressing Fos after mating are GABAergic and 27% are putatively glutamatergic, so the ones that contain NOS (36%) could be wholly among those making GABA or part of both other subsets. However, if those three subsets do not overlap, all lateral MeApd cells activated with mating could be accounted for by cells making GABA, glutamate, or NO.
NOS cell distribution and co-localization with AR
NOS cell distribution in the gerbil forebrain resembles that in rats (Vincent and Kimura, 1992; McDonald et al., 1993; Yamada et al., 1996; Sato et al., 2005) and mice (Scordalakes et al., 2002) but differs from that in hamsters (Hadeshi and Wood, 1996) and monkeys (Satoh et al., 1995) in that gerbils have NOS cells in the supraoptic and ventromedial hypothalamic nuclei, PVH, and AHi. NOS cells are absent in the ventromedial hypothalamic nucleus and AHi of hamsters, in the PVH of monkeys, and in the supraoptic nucleus of both these species. NOS cells in the gerbil PdPN match descriptions of those seen in rats in the PdPN (Sato et al., 2005) and rostral AC/ PdPN (Vanhatalo and Soinila, 1995; Yamada et al., 1996).
NOS cells with AR are common in gerbils in brain regions where they are prevalent in hamsters (Hadeshi and Wood, 1996), rats (Yokosuka and Hayashi, 1996; Sato et al., 2005), and mice (Scordalakes et al., 2002), such as in the ventral premammillary nucleus, the MPN/ MPOA, and the MeApd. NOS-AR co-localization had not previously been studied in the lateral MeApd (Vincent and Kimura, 1992; McDonald et al., 1993; Satoh et al., 1995; Hadeshi and Wood, 1996; Yamada et al., 1996; Scordalakes et al., 2002), and was reportedly sparse in the rat PdPN (Sato et al., 2005). Gerbils also have many AR-containing NOS cells in the BST, anterior MeA, and cortical nucleus of the amygdala, where hamsters have few or none (Hadeshi and Wood, 1996). These distribution discrepancies may reflect procedural rather than species differences, though, because NADPH-d histochemistry preceded AR ICC here but followed it in the work on hamsters (Hadeshi and Wood, 1996). Comparing the percentages of AR-containing NOS cells seen here in subdivisions of the gerbil MPN and MeApd with those seen overall in the mouse MPOA and MeApd (Scordalakes et al., 2002) reveals similarities (25% in gerbil MPNm; 21% in mouse MPOA) and differences (69% in gerbil lateral MeApd; 20% in mouse MeApd).
The difference in the MeApd could reflect regional or species differences or the fact that we allowed NOS staining to continue until cells in the PdPN and lateral MeApd were visible. That may also have contributed to our seeing more NOS-AR co-localization in the PdPN than was seen in rats (Sato et al., 2005). Using every other 30-μm section versus every fourth 40-μm section probably also increased our opportunity to observe the PdPN, given its size (≈150-μm diameter).
Effects of androgens on NOS cells
The decrease in NOS cell staining in the gerbil MPNm and PdPN after castration, and the ability of T, but not DHT, to prevent this decline, are consistent with data on the MPN of rats and hamsters (Hadeshi and Wood, 1996; Du and Hull, 1999; Putnam et al., 2005; Sato et al., 2005). The POA region (rostral periventricular) that plays the same role in regulating mating in male whiptail lizards shows the same responses (Sanderson et al., 2008). In contrast, in homogenates of thicker (1-mm) coronal sections through the rat brain at the rostrocaudal level of the MPNc, NOS activity and mRNA in the area between the ac and optic chiasm increased after castration and decreased with exposure to T or DHT (Singh et al., 1999). We concur with Sanderson et al. (2008) that those homogenates may have combined NOS cells with different responses to androgens such that the net response differed from that seen in any of the more discrete areas studied to date. Singh et al. (1999) obtained similar responses to castration and DHT when they counted NOS cells in a strip of tissue just below the ac at the level of the MPNc. Although that strip may have overlapped the PdPN, it included cells from other POA subdivisions and the BST. When Singh et al. (1999) studied the MPNc and its immediate surround, they found few NOS cells, consistent with our findings for the gerbil MPNc, and no effect of castration. The castration-related effect that we saw in the MPNc involved NOS fibers, and the hormonal effects we saw on MPNm NOS cells were from an area ventromedial to the MPNc.
The decrease in NOS fibers in the gerbil MPNc after castration may be secondary to the decrease in NOS cells seen in the caudal BSTpr, because the caudal BSTpr projects to the MPNc (De Vries et al., 1988). A decrease in BSTpr NOS cells was also seen in castrated male rats but not consistently (Du and Hull, 1999). This may reflect a varying response over time or variations across tissue processing runs. Our analysis showed that the latter effects can be substantial.
The decrease in NOS cells seen here after castration in the AHi and lateral MeApd has not been described before. No effect of castration was seen in the MeApd of rats and hamsters (Hadeshi and Wood, 1996; Du and Hull, 1999); however, the area studied here represents only a fraction of the total MeApd and has distinctive properties, as reflected in its activation after ejaculation. In contrast, castration increased NOS activity in a homogenate of rat ventrolateral forebrain that included parts of the amygdala rostral to the MeA (Singh et al., 1999). Again, this may reflect inclusion of different NOS cell types within and outside the amygdala. Differing responses among cell types in the MeApd may explain why castration did not affect NOS cell somal size in the lateral MeApd of gerbils but shrank the somas of Nissl-stained MeApd cells in rats (Cooke et al., 1999).
For NOS cells in the gerbil PdPN, the percentage that disappeared after castration (49%) was similar to the percentage with AR (46%), suggesting that the cells with AR may be the ones affected by castration. Because the castration-induced decrease in PdPN NOS cells was prevented by T but not DHT, T may affect those cells without undergoing metabolism (Askew et al., 2007). Alternatively, T may act via estradiol and an estrogen receptor, as in the (M)POA of rats and mice (Scordalakes et al., 2002; Putman et al., 2005), or indirectly via T-sensitive afferents, efferents, or neighbors. For NOS cells in the MPNm, the decline after castration (60%) was much larger than the percentage with AR (25%), suggesting that at least some effects of T on NOS cells there occur via another receptor or other cells.
In conclusion, the current study indicates that NO may synchronize the activation of PdPN and lateral MeApd neurons at ejaculation and, in the MPNm, may participate in multiple aspects of mating. T regulation of mating may be mediated in part by actions on NOergic cells in the PdPN, MPNm, and lateral MeApd via direct binding to AR or via metabolism to estradiol. Because a large portion of ejaculation-related cells in the PdPN and lateral MeApd contain NO, these results also suggest that the functions of these cells may be elucidated by using selective manipulation of NOergic transmission.
Acknowledgments
Grant sponsor: National Institute of Mental Health; Grant numbers: MH26481; Training Grant MH14599.
Dr. Gail Prins kindly provided the androgen receptor antibody. This work was submitted by D.A.S. in partial satisfaction of the requirements for the Ph.D. in Biological Sciences.
Abbreviations
- ac
anterior commissure
- AC
anterior commissural nucleus
- AHi
amygdalohippocampal area
- AR
androgen receptor
- AVPv
anteroventral periventricular nucleus of the hypothalamus
- BSTpr
bed nuclei of the stria terminalis (principal)
- DHT
dihydrotestosterone
- fx
fornix
- MeApd
medial nucleus of the amygdala (posterodorsal)
- MPNc, l, m
medial preoptic nucleus (central, lateral, medial)
- MPOA
medial preoptic area
- NADPH-d
nicotinamide adenine dinucleotide phosphate diaphorase
- NOS
nitric oxide synthase
- ot
optic tract
- PdPN
posterodorsal preoptic nucleus
- POA
preoptic area
- PVH
paraventricular nucleus of the hypothalamus
- VMH
ventromedial hypothalamic nucleus of the hypothalamus
LITERATURE CITED
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Askew EB, Gampe RT, Jr, Stanley TB, Faggart JL, Wilson EM. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J Biol Chem. 2007;282:25801–25816. doi: 10.1074/jbc.M703268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum MJ, Everitt BJ. Increased expression of c-fos in the medial preoptic area after mating in male rats: role of afferent inputs from the medial amygdala and midbrain central tegmental field. Neuroscience. 1992;50:627–646. doi: 10.1016/0306-4522(92)90452-8. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JTM. Copulation in castrated male rats following combined treatment with estradiol and dihy-drotestosterone. Science. 1973;182:283–285. doi: 10.1126/science.182.4109.283. [DOI] [PubMed] [Google Scholar]
- Bhat GK, Mahesh VB, Lamar CA, Ping L, Aguan K, Brann DW. Histochemical localization of nitric oxide neurons in the hypothalamus: association with gonadotropin-releasing hormone neurons and co-localization with N-methyl-D aspartate receptors. Neuroendocrinology. 1995;62:187–197. doi: 10.1159/000127004. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Dixen HR, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Chikada N, Imaki T, Seki T, Harada S, Nakajima K, Yoshimoto T, Naruse M, Demura H, Minami S, Takano K. Distribution of c-fos mRNA in the brain following intracerebro-ventricular injection of nitric oxide (NO)-releasing compounds: possible role of NO in central cardiovascular regulation. J Neuroendocrinol. 2000;12:1112–1123. doi: 10.1046/j.1365-2826.2000.00561.x. [DOI] [PubMed] [Google Scholar]
- Clark MM, Bishop AM, vom Saal FS, Galef BG. Responsiveness to testosterone of male gerbils from known intra-uterine positions. Physiol Behav. 1993;53:1183–1187. doi: 10.1016/0031-9384(93)90377-r. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984;224:132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- Commins D, Yahr P. Autoradiographic localization of estrogen and androgen receptors in the sexually dimorphic area and other regions of the gerbil brain. J Comp Neurol. 1985;231:473–489. doi: 10.1002/cne.902310406. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci U S A. 1999;96:7538–7540. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen LM, Peters HJPW, Veening JG. Fos-immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- Coquelin A, Bronson FH. Secretion of luteinizing hormone in male mice: factors that influence release during sexual encounters. Endocrinology. 1980;106:1224–1229. doi: 10.1210/endo-106-4-1224. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ, Gonzales CL, Yahr P. Afferent connections of the sexually dimorphic area of the hypothalamus in male and female gerbils. J Comp Neurol. 1988;271:91–105. doi: 10.1002/cne.902710110. [DOI] [PubMed] [Google Scholar]
- Dinerman JL, Dawson TM, Schell MJ, Snowman A, Snyder SH. Endothelial nitric oxide synthase localized to hippocampal pyramidal cells: implications for synaptic plasticity. Proc Natl Acad Sci U S A. 1994;91:4214–4218. doi: 10.1073/pnas.91.10.4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JM, Brann JH, Gil M, Hull EM. Sexual experience increases nitric oxide synthesis in the medial pre-optic area of male rats. Behav Neurosci. 2006;120:1389–1394. doi: 10.1037/0735-7044.120.6.1389. [DOI] [PubMed] [Google Scholar]
- Du J, Hull EM. Effects of testosterone on neuronal nitric oxide synthase and tyrosine hydroxylase. Brain Res. 1999;836:90–98. doi: 10.1016/s0006-8993(99)01618-2. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Ferrini MG, Magee TR, Vernet D, Rajfer J, Gonzalez-Cadivid NF. Penile neuronal nitric oxide synthase and its regulatory proteins are present in hypothalamic and spinal cord regions involved in the control of penile erection. J Comp Neurol. 2003;458:48–61. doi: 10.1002/cne.10543. [DOI] [PubMed] [Google Scholar]
- Finn PD, Yahr P. Projection from the ventral bed nucleus of the stria terminalis to the retrorubral field in rats and the effects of cells in these areas on mating in male rats versus gerbils. Horm Behav. 2005;47:123–138. doi: 10.1016/j.yhbeh.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Finn PD, De Vries GJ, Yahr P. Efferent projections of the sexually dimorphic area of the gerbil hypothalamus: anterograde identification and retrograde verification in males and females. J Comp Neurol. 1993;338:491–520. doi: 10.1002/cne.903380403. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Dahlstrom A, Hoistad M, Marcellino D, Jansson A, Rivera A, Diaz-Cabiale Z, Jacobsen K, Tinner-Staines B, Hagman B, Leo G, Staines W, Guidolin D, Kehr J, Genedani S, Belluardo N, Agnati LF. From the Golgi-Cajal mapping to the transmitter-based characterization of the neuronal networks leading to two modes of brain communication: wiring and volume transmission. Brain Res Rev. 2007;55:17–54. doi: 10.1016/j.brainresrev.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Gabbot PLA, Bacon SJ. Histochemical localization of NADPH-dependent diaphorase (nitric oxide synthase) activity in vascular endothelial cells in the rat brain. Neuroscience. 1993;57:79–95. doi: 10.1016/0306-4522(93)90113-t. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Boulton CL. Nitric oxide signaling in the central nervous system. Annu Rev Physiol. 1995;57:683–706. doi: 10.1146/annurev.ph.57.030195.003343. [DOI] [PubMed] [Google Scholar]
- Gudi T, Casteel DE, Vinson C, Boss GR, Pilz RB. NO activation of fos promoter elements requires nuclear translocation of G-kinase I and CREB phosphorylation but is independent of MAP kinase activation. Oncogene. 2000;19:6324–6333. doi: 10.1038/sj.onc.1204007. [DOI] [PubMed] [Google Scholar]
- Haby C, Lisovoski F, Aunis D, Zwiller J. Stimulation of the cyclic GMP pathway by NO induces expression of the immediate early genes c-fos and junB in PC12 cells. J Neurochem. 1994;62:496–501. doi: 10.1046/j.1471-4159.1994.62020496.x. [DOI] [PubMed] [Google Scholar]
- Hadeshi Y, Wood RI. Nitric oxide synthase in mating behavior circuitry of male Syrian hamster brain. J Neurobiol. 1996;30:480–492. doi: 10.1002/(SICI)1097-4695(199608)30:4<480::AID-NEU4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. C-fos immunoreactivity in the sexually dimorphic area of the hypothalamus and related brain regions of male gerbils after exposure to sex-related stimuli or performance of specific sexual behaviors. Neuroscience. 1996;72:1049–1071. doi: 10.1016/0306-4522(95)00602-8. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Cell-body lesions of the posterodorsal preoptic nucleus or posterodorsal medial amygdala, but not the parvicellular subparafascicular thalamus, disrupt mating in male gerbils. Physiol Behav. 2000;68:317–331. doi: 10.1016/s0031-9384(99)00182-1. [DOI] [PubMed] [Google Scholar]
- Heeb MM, Yahr P. Anatomical and functional connections among cell groups in the gerbil brain that are activated with ejaculation. J Comp Neurol. 2001;439:248–258. doi: 10.1002/cne.1346. [DOI] [PubMed] [Google Scholar]
- Hope BT, Michael GJ, Knigge KM, Vincent SR. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991;88:2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AM, Everitt BJ, Lightman SL, Todd K. Oxytocin in the central nervous system and sexual behavior in male rats. Brain Res. 1987;414:133–137. doi: 10.1016/0006-8993(87)91333-3. [DOI] [PubMed] [Google Scholar]
- Hwang S-J, Huh Y. Age-related changes in nitric oxide synthase in the lateral geniculate nucleus of rats. J Molec Hist. 2010;41:129–135. doi: 10.1007/s10735-010-9268-4. [DOI] [PubMed] [Google Scholar]
- Ju G, Swanson LW. Studies on the cellular architecture of the bed nuclei of the stria terminalis in the rat: I. Cytoarchitecture. J Comp Neurol. 1989;280:587–602. doi: 10.1002/cne.902800409. [DOI] [PubMed] [Google Scholar]
- Kadekaro M, Terrell ML, Liu H, Gestl S, Bui V, Summy-Long JY. Effects of L-NAME on cerebral metabolic, vasopressin, oxytocin, and blood pressure responses in hemorrhaged rats. Am J Physiol. 1998;274:R1070–1077. doi: 10.1152/ajpregu.1998.274.4.R1070. [DOI] [PubMed] [Google Scholar]
- Kadowaki K, Kishimoto J, Leng G, Emson PC. Upregulation of nitric oxide synthase (NOS) gene expression together with NOS activity in the rat hypothalamic-hypophysial system after chronic salt loading: evidence for a neuromodulatory role of nitric oxide in arginine vasopressin and oxytocin secretion. Endocrinology. 1994;134:1011–1017. doi: 10.1210/endo.134.3.7509733. [DOI] [PubMed] [Google Scholar]
- Kamel F, Mock EJ, Wright WW, Frankel AI. Alterations in plasma concentrations of testosterone, LH, and prolactin associated with mating in the male rat. Horm Behav. 1975;6:277–288. doi: 10.1016/0018-506x(75)90014-8. [DOI] [PubMed] [Google Scholar]
- Lagoda G, Muschamp JW, Vigdorchik A, Hull EM. A nitric oxide synthesis inhibitor in the medial preoptic area inhibits copulation and stimulus sensitization in male rats. Behav Neurosci. 2004;118:1317–1323. doi: 10.1037/0735-7044.118.6.1317. [DOI] [PubMed] [Google Scholar]
- Liu Y-C, Salamone JD, Sachs BD. Impaired sexual response after lesions of the paraventricular nucleus of the hypothalamus in male rats. Behav Neurosci. 1997;111:1361–1367. doi: 10.1037//0735-7044.111.6.1361. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Matuszewich L, Howard RV, Du J, Hull EM. Nitric oxide promotes medial preoptic dopamine release during male rat copulation. NeuroReport. 1996;8:31–34. doi: 10.1097/00001756-199612200-00007. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Mannella P, Sanchez AM, Giretti MS, Genazzani AR, Simoncini T. Oestrogen and progestins differently prevent glutamate toxicity in cortical neurons depending on prior hormonal exposure via the induction of neural nitric oxide synthase. Steroids. 2009;74:650–656. doi: 10.1016/j.steroids.2009.02.011. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Payne DR, Mascagni F. Identification of putative nitric oxide producing neurons in the rat amygdala using NADPH-diaphorase histochemistry. Neuroscience. 1993;52:97–106. doi: 10.1016/0306-4522(93)90185-i. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Mauri A, Argiolas A. Nitric oxide production is increased in the paraventricular nucleus of the hypothalamus of male rats during non-contact penile erections and copulation. Eur J Neurosci. 1998;10:1968–1974. doi: 10.1046/j.1460-9568.1998.00207.x. [DOI] [PubMed] [Google Scholar]
- Moretto M, Lopez FJ, Negro-Vilar A. Nitric oxide regulates luteinizing-releasing hormone secretion. Endocrinology. 1993;133:2399–2402. doi: 10.1210/endo.133.5.8104781. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Secki JR, Burton S, Checkley SA, Lightman SL. Changes in oxytocin and vasopressin secretion during sexual activity in men. J Clin Endocrinol Metab. 1987;65:738–741. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- Ota M, Crofton JT, Festavan GT, Share L. Evidence that nitric oxide can act centrally to stimulate vasopressin release. Neuroendocrinology. 1993;57:955–959. doi: 10.1159/000126459. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2. Sydney: Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 3. San Diego: Academic Press; 1997. [DOI] [PubMed] [Google Scholar]
- Preston GA, Lyon TT, Yin Y, Lang JE, Solomon GY, Annab L, Srinivasan DG, Alcorta DA, Barrett JC. Induction of apoptosis by c-Fos protein. Mol Cell Biol. 1996;16:211–218. doi: 10.1128/mcb.16.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins GS, Birch L, Greene GL. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- Pu S, Xu B, Kalra SP, Kalra PS. Evidence that gonadal steroids modulate nitric oxide efflux in the medial preoptic area: effects of N-methyl-D-aspartate and correlation with luteinizing hormone secretion. Endocrinology. 1996;137:1949–1955. doi: 10.1210/endo.137.5.8612535. [DOI] [PubMed] [Google Scholar]
- Putnam SK, Sato S, Riolo JV, Hull EM. Effects of testosterone metabolites on copulation, medial preoptic dopamine, and NOS-immunoreactivity in castrated male rats. Horm Behav. 2005;47:513–522. doi: 10.1016/j.yhbeh.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Rettori V, Belova N, Dees WL, Nyberg CL, Gimeno M, McCann SM. Role of nitric oxide in the control of luteinizing hormone-releasing hormone release in vivo and in vitro. Proc Natl Acad Sci U S A. 1993;90:10130–10134. doi: 10.1073/pnas.90.21.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson NSR, Le B, Zhou Z, Crews D. Preoptic neuronal nitric oxide synthase induction by testosterone is consistent with a role in gating male copulatory behavior. Eur J Neurosci. 2008;27:183–190. doi: 10.1111/j.1460-9568.2007.05989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato SM, Wersinger SR, Hull EM. The effects of nitric oxide-cGMP pathway stimulation on dopamine in the medial preoptic area and copulation in DHT-treated castrated male rats. Horm Behav. 2007;52:177–182. doi: 10.1016/j.yhbeh.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Braham CS, Putnam SK, Hull EM. Neuronal nitric oxide synthase and gonadal steroid interaction in the MPOA of male rats: colocalization of and testosterone-induced restoration of copulation and nNOS-immunoreactivity. Brain Res. 2005;1043:205–213. doi: 10.1016/j.brainres.2005.02.074. [DOI] [PubMed] [Google Scholar]
- Sato Y, Horita H, Kurohata T, Adachi H, Tsukamoto T. Effect of the nitric oxide level in the medial preoptic area on male copulatory behavior in rats. Am J Physiol. 1998;274:R243–R247. doi: 10.1152/ajpregu.1998.274.1.R243. [DOI] [PubMed] [Google Scholar]
- Sato Y, Christ GJ, Horita H, Adachi H, Suzuki N, Tsukamoto T. The effects of alterations in nitric oxide levels in the paraventricular nucleus on copulatory behavior and reflexive erections in male rats. J Urol. 1999;162:2182–2185. doi: 10.1016/S0022-5347(05)68156-6. [DOI] [PubMed] [Google Scholar]
- Satoh K, Arai R, Ikemoto K, Narita M, Nagai T, Ohshima H, Kitahama K. Distribution of nitric oxide synthase in the central nervous system of Macaca fuscata: subcortical regions. Neuroscience. 1995;66:685–696. doi: 10.1016/0306-4522(95)00040-p. [DOI] [PubMed] [Google Scholar]
- Sayag N, Hoffman NW, Yahr P. Telencephalic connections of the sexually dimorphic area of the gerbil hypothalamus that influence male sexual behavior. Behav Neurosci. 1994;108:743–757. doi: 10.1037//0735-7044.108.4.743. [DOI] [PubMed] [Google Scholar]
- Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor α and androgen receptor in the regulation of neuronal nitric oxide synthase. J Comp Neurol. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- Serrats J, Sawchenko PE. CNS activational responses to staphylococcal enterotoxin B: T-lymphocyte-dependent immune challenge effects on stress-related circuitry. J Comp Neurol. 2006;495:236–254. doi: 10.1002/cne.20872. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. Projections of the posterodorsal preoptic nucleus and the lateral part of the posterodorsal medial amygdala in male gerbils, with emphasis on cells activated with ejaculation. J Comp Neurol. 2002;444:74–94. doi: 10.1002/cne.10128. [DOI] [PubMed] [Google Scholar]
- Simmons DA, Yahr P. GABA and glutamate in mating-activated cells in the preoptic area and medial amygdala of male gerbils. J Comp Neurol. 2003;459:290–300. doi: 10.1002/cne.10605. [DOI] [PubMed] [Google Scholar]
- Singh R, Pervin S, Shryne J, Gorski R, Chaudhuri G. Castration increases and androgens decrease nitric oxide synthase activity in the brain: physiologic implications. Proc Natl Acad Sci U S A. 2000;97:3672–3677. doi: 10.1073/pnas.050583297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RAJ, Mistry R, Haustein MD, Griffin SJ, Tong H, Graham BP, Forsythe ID. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Brain maps: structure of the rat brain. Amsterdam: Elsevier; 1992. [Google Scholar]
- Tanaka M, Ikeda T, Hayashi S, Iijima N, Amaya F, Hisa Y, Ibata Y. Nitrergic neurons in the medial amygdala project to the hypothalamic paraventricular nucleus of the rat. Brain Res. 1997;777:13–21. doi: 10.1016/s0006-8993(97)00948-7. [DOI] [PubMed] [Google Scholar]
- Todd K, Lightman SL. Oxytocin release during coitus in male and female rabbits: effect of opiate receptor blockade with naloxone. Psychoneuroendocrinology. 1986;11:367–371. doi: 10.1016/0306-4530(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Tomimoto H, Akiguchi I, Wakita H, Nakamura S, Kimura J. Histochemical demonstration of membranous localization of endothelial nitric oxide synthase in endothelial cells of the rat brain. Brain Res. 1994;667:107–110. doi: 10.1016/0006-8993(94)91718-3. [DOI] [PubMed] [Google Scholar]
- Ulibarri CM, Yahr P. Ontogeny of the sexually dimorphic area of the gerbil hypothalamus. Dev Brain Res. 1993;74:14–24. doi: 10.1016/0165-3806(93)90078-o. [DOI] [PubMed] [Google Scholar]
- Ulibarri C, Yahr P. Effects of androgens and estrogens on sexual differentiation of sex behavior, scent marking, and the sexually dimorphic area of the gerbil hypothalamus. Horm Behav. 1996;30:107–130. doi: 10.1006/hbeh.1996.0015. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Soinila S. Nitric oxide synthase in the hypothalamopituitary pathways. J Chem Neuroanat. 1995;8:165–173. doi: 10.1016/0891-0618(94)00043-s. [DOI] [PubMed] [Google Scholar]
- Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- Yahr P, Gregory JE. The medial and lateral cell groups of the sexually dimorphic area of the gerbil hypothalamus are essential for male sex behavior and act via separate pathways. Brain Res. 1993;631:287–296. doi: 10.1016/0006-8993(93)91547-6. [DOI] [PubMed] [Google Scholar]
- Yahr P, Stephens DR. Hormonal control of sexual and scent marking behaviors of male gerbils in relation to the sexually dimorphic area of the hypothalamus. Horm Behav. 1987;21:331–346. doi: 10.1016/0018-506x(87)90018-3. [DOI] [PubMed] [Google Scholar]
- Yahr P, Newman A, Stephens DR. Sexual behavior and scent marking in male gerbils: comparison of changes after castration and testosterone replacement. Horm Behav. 1979;13:174–184. doi: 10.1016/0018-506x(79)90056-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Emson P, Hokfelt T. Immunohistochemical mapping of nitric oxide synthase in the rat hypothalamus and colocalization with neuropeptides. J Chem Neuroanat. 1996;10:295–316. doi: 10.1016/0891-0618(96)00133-0. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, Hayashi S. Colocalization of neuronal nitric oxide synthase and androgen receptor immunoreactivity in the premammillary nucleus in rats. Neurosci Res. 1996;26:309–314. doi: 10.1016/s0168-0102(96)01109-1. [DOI] [PubMed] [Google Scholar]