Abstract
Salts play a mysterious role in desorption mass spectrometry, especially in biological samples.[1] We used trehalose films doped with a peptide as a well defined model system to investigate the ionization effects in organic molecular depth profiling. Sodium salts at 1% level were added into the solution used to produce the trehalose films, and depth profiles were obtained with a C60 ion source. The results show that the protonated molecular ion signal from the peptide and the quasimolecular ion signal of trehalose are significantly suppressed by the addition of salts, whereas the signals representing salt clusters and salt adducts of trehalose are formed in both positive and negative modes. The formation of protonated molecular ions is found to correlate with the ratio between protonated and bare water ions, suggesting that the latter can be used as an indicator for the accumulation of protons liberated by the ion bombardment. In experiments where no salt was added, it is shown that the surface variation of the protonated molecular ion signal strongly depends upon the water content of the trehalose film.
Keywords: molecular depth profiling, SIMS, C60, trehalose film, ionization, cluster ions
Introduction
SIMS is finding more applications in chemical imaging of biological samples.[2] With the development of cluster ion sources, it has been shown that it is possible to identify the lateral distribution of molecular species on and within the surface of cells and tissues.[3,4] Therefore, SIMS bioimaging can be expanded from two-dimension (2D) analysis to three-dimension (3D) analysis.[3,4]
One of the challenges in mass spectrometry bioimaging is obtaining useful biological molecular information in a complicated environment. The matrix of biological samples has great influence on the ionization of analytes.[5] Studies have shown that the yield of protonated species can be enhanced in hydrated environment by C60 bombardment.[6,7] In particular, it has been suggested that the formation efficiency of molecular secondary [M+H]+ ions or specific fragments derived from these may correlate with the liberation of protons by bombardment-induced dissociation of water molecules.[7]
Moreover, suppression or enhancement of the ion formation related to salts in tissue samples has been observed in SIMS as well as in matrix-assisted laser desorption ionization (MALDI) experiments.[4,5] Depth profiling of brain tissue by a 40 keV buckminsterfullerene (C60) primary ion beam showed a rapid loss of molecular signals from lipid and protein fragments during sputtering, accompanied by the increase of signals from sodium and potassium adducts of phosphate and protein fragments.[4] Piwowar et al. investigated the effect of doping a series of alkali chlorides into an arginine matrix.[1] Their data acquired on the arginine film surface indicate that the signal decrease of positive ions is likely caused by a neutralization process involving counter-chloride anions.[1]
Our previous work has shown that spin-cast peptide-doped trehalose films are a reliable platform to perform depth profiling and can provide valuable parameters leading to 3D imaging characterization of biomaterials.[8] Here we used a trehalose film doped with peptide as a model system to study ionization effects during the sputtering with a 40 keVC60 ionsource. Theresults show that the formation of protonated molecular species is strongly influenced by the water content of the deposited films. It is also found that the addition of salt influences the total molecular ion intensity of trehalose matrix and peptide dopant in a very different way.
Experimental
Trehalose, peptide Gly–Gly–Tyr–Arg (GGYR) and sodium chloride (NaCl) were purchased from Sigma-Aldrich Co. (St Louis, MO) and used without further purification. The water used in preparation of all films was purified by a Nanopure Diamond Life Science Ultrapure Water System (Barnstead International, Dubuque, IA) and had a resistivity of at least 18.2 MΩ-cm. Presliced 5 mm × 5 mm single crystal (100) silicon wafers were supplied by Ted Pella Inc. (Redding, CA). The film preparation process has been described elsewhere.[8] Films doped with sodium chloride were prepared by trehalose peptide solutions containing 1% NaCl by weight of solid.
The depth profiling was performed in a ToF-SIMS instrument equipped with a 40 keV C60 source (IOG 40–60, Ionoptika; Southampton, UK), directed at a 40° angle relative to the surface normal. For depth profiling, the C60+ ion beam was operated in d.c. mode to erode through the film at an area of 350 μm × 450 μm in 3 s intervals. Between erosion cycles, TOF mass spectra were acquired at an area of 250 μm × 350 μm inside the sputtered region using the pulsed C60+ projectile beam at an ion fluence of 1010 cm−2. The crater depths were measured by a KLA-Tencor Nanopics 2100 atomic force microscopy (AFM) in contact mode.
Results and Discussion
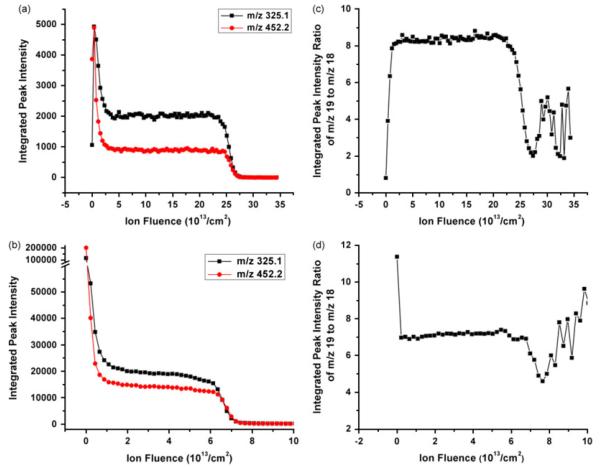
We begin the discussion of ionization effects by looking at the initial stages of a depth profile obtained on a clean, salt-free trehalose film. The profile obtained in our earlier work[8] as shown in Fig. 1a, reveals that there is an initial increase of the quasimolecular ion signal [M–OH]+ of the trehalose at 325.1 m/z and molecular ion signal [M+H]+ of peptide dopant at 452.2 m/z. Both the molecular ions are formed through protonation of the parent molecule, and [M–OH]+ ions are the products of the protonated trehalose molecules losing a water molecule.
Figure 1.
Molecular ion signals of the trehalose (325.1 m/z) and peptide dopant molecule (452.2 m/z) from film A and B are plotted vs ion fluence in a and b. Signal ratios of protonated (19 m/z) and nonprotonated (18 m/z) water molecules from film A and B are plotted vs C60+ ion fluence in c and d.
As explained elsewhere in this volume,[9] the initial transient is attributed to an increase of the ionization efficiency superimposed by an exponential decay of the molecule ejection yield induced by damage accumulation. As seen in Fig. 1b, however, this effect is not always observed, even though the films analyzed in both cases have been deposited in the same way. To investigate the cause of this deviation, we take the signal intensity ratio of protonated (19 m/z) and nonprotonated (18 m/z) water molecules as an indicator for the availability of free protons liberated by the ion bombardment, which may promote the formation of the observed molecular ions.[6,7] As shown in Fig. 1c, it is indeed found that the increased ionization efficiency in film A is correlated with an increase of the 19/18 ratio by roughly a factor of 10. In contrast, a much less pronounced variation is observed for film B, and at the same time the 19/18 ratio is found to decrease (Fig. 1d). This finding is consistent with the fact that the ionization efficiency does not seem to increase in this case. Looking at the total intensity at 18 and 19 m/z compared to that of both the molecular ions at the beginning of the profile and the Si signal at 28 m/z measured after removal of the entire film, one finds that film A contains about a factor of 5–10 more water than film B. Apparently, the water content of the deposited trehalose film critically influences the surface variation of the ionization efficiency, supporting the notion that the bombardment-induced dissociation of water might be a means to enhance the formation of protonated molecular ions.
In order to examine how the salt affects the depth profiling of a trehalose film doped with peptide, 1% NaCl by weight of solid was added during the film preparation. The sputter rate of both films was obtained from AFM data. Although it has been observed that doping of different peptides at the percent level can significantly change the sputter yield of the trehalose film,[8] NaCl does not seem to have the same effect, and the sputter yield remains practically the same after 1% NaCl is added. As shown in Fig. 2, similar depth profiles are obtained from the salt-free film and 1% NaCl-doped film. The signal intensities of [M–OH]+ and [M+H]+, however, are both suppressed by the same amount upon the addition of NaCl. At the beginning of the depth profile, the molecular ion signals are reduced by a factor of 2, while in the steady state the signals are suppressed by a factor of 10. Since the ratio between initial and steady state signal reflects the cleanup efficiency,[10] i.e. the ratio between the number of sputtered and damaged molecules per projectile impact, this finding indicates that the presence of NaCl must enhance the damage cross-section during the ion bombardment.
Figure 2.
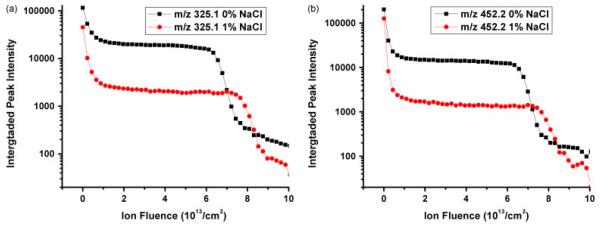
Depth profiles of trehalose film doped with GGYR and 0% and 1% NaCl. Secondary ion intensities of trehalose at 325.1 m/z and GGYR at 452.2 m/z are plotted vs C60+ ion fluence respectively in a and b.
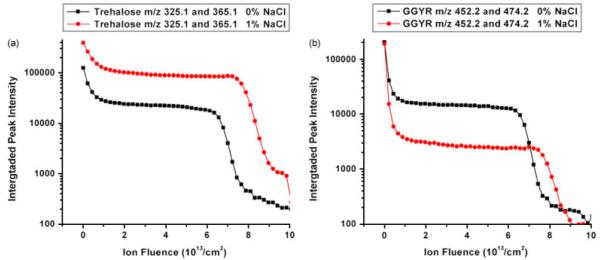
As cation adducts are commonly observed in MALDI experiments from biological samples,[5] sodium cation adducts of trehalose and GGYR are found in the spectrum obtained from the NaCl-doped films. Besides sodium cation adducts, chlorine anion adduct peaks also show up in the negative ion spectrum. However, the presence of NaCl induces different behavior of the trehalose and GGYR peptide signals. With NaCl present, the total secondary ion intensity of trehalose quasimolecular ions [M–OH]+ and sodium adducts [M+Na]+ is enhanced more than three times mainly by the prominent formation of [M+Na]+, as shown in Fig. 3a. Although the existence of [M+Na]+ of GGYR is also detected, its intensity from NaCl-doped film is only 10% of the intensity of [M+H]+ from salt-free film. Consequently, the total secondary ion intensity of GGYR [M+H]+ and [M+Na]+ is found to decrease by more than 70% in the NaCl-doped film (Fig. 3b). It is obvious that the trehalose molecule is prone to binding to a sodium ion, while GGYR is not. As observed in both SIMS and MALDI experiments, the formation of sodium-attached ions is dependent upon the chemical structure of the analyte molecule.[11] Compared to GGYR, trehalose has eight hydroxyl groups available to combine with sodium cations. Piwowar et al. have found that the arginine molecular ion was neutralized by chloride counteranions.[1] One might therefore speculate, that the GGYR molecular ion may also exhibit a similar behavior and have a higher binding efficiency for Cl− instead of Na+.
Figure 3.
Total secondary ion intensities of trehalose molecular ions [M–OH]+ at 325.1 m/z and sodium adducts [M+Na]+ at 365.1 m/z from the films doped with 0 and 1% NaCl are plotted vs C60+ ion fluence in a. Total secondary ion intensities of GGYR molecular ions[M+H]+ at 452.2 m/z and sodium adducts [M+Na]+ at 474.2 m/z from the films doped with 0 and 1% NaCl are plotted vs C60+ ion fluence in b.
Conclusion
Using peptide-doped trehalose films as a model system, we have shown that the molecular ionization efficiency is affected by the water content of the sample film. This behavior is attributed to the enhanced availability of protons due to ion bombardment-induced dissociation of water molecules. The presence of salt in the film affects the ionization of different organic molecules in completely different ways. In the case of trehalose, the total ionization efficiency of the ejected molecules is enhanced by forming sodium adducts, while a strong decrease of the molecular ionization efficiency is observed for the GGYR peptide in a trehalose matrix.
Acknowledgements
The authors would like to thank Dr Juan Cheng for her contributions to the presented research. Financial support from the National Institute of Health under GrantNo. 2R01 EB002016-16 and the National Science Foundation under Grant No. CHE-0908226 are acknowledged.
References
- [1].Piwowar AM, Lockyer NP, Vickerman JC. Anal. Chem. 2009;81:1040. doi: 10.1021/ac8020888. [DOI] [PubMed] [Google Scholar]
- [2].Winograd N. Anal. Chem. 2005;77:142a. doi: 10.1021/ac051263k. [DOI] [PubMed] [Google Scholar]
- [3].Fletcher JS, Lockyer NP, Vaidyanathan S, Vickerman JC. Anal. Chem. 2007;79:2199. doi: 10.1021/ac061370u. [DOI] [PubMed] [Google Scholar]
- [4].Jones EA, Lockyer NP, Vickerman JC. Anal. Chem. 2008;80:2125. doi: 10.1021/ac702127q. [DOI] [PubMed] [Google Scholar]
- [5].Chen WY, Chen YC. Anal. Chem. 2007;79:8061. doi: 10.1021/ac0709450. [DOI] [PubMed] [Google Scholar]
- [6].Weibel DE, Lockyer N, Vickerman JC. Appl. Surf. Sci. 2004;146:231–232. [Google Scholar]
- [7].Conlan XA, Lockyer NP, Vickerman JC. Rapid Commun. Mass Spectrom. 2006;20:1327. doi: 10.1002/rcm.2446. [DOI] [PubMed] [Google Scholar]
- [8].Cheng J, Winograd N. Anal. Chem. 2005;77:3651. doi: 10.1021/ac048131w. [DOI] [PubMed] [Google Scholar]
- [9].Willingham D, Brenes DA, Wucher A, Winograd N. J. Phys. Chem. C. 2010;114:5391. doi: 10.1021/jp9054632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Wucher A, Cheng J, Winograd N. J. Phys. Chem. C. 2008;112:16550. doi: 10.1021/jp8049763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hanton SD, Clark PAC, Owens KG. J. Am. Soc. Mass Spectrom. 1999;10:104. [Google Scholar]