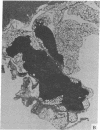
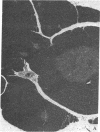
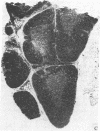
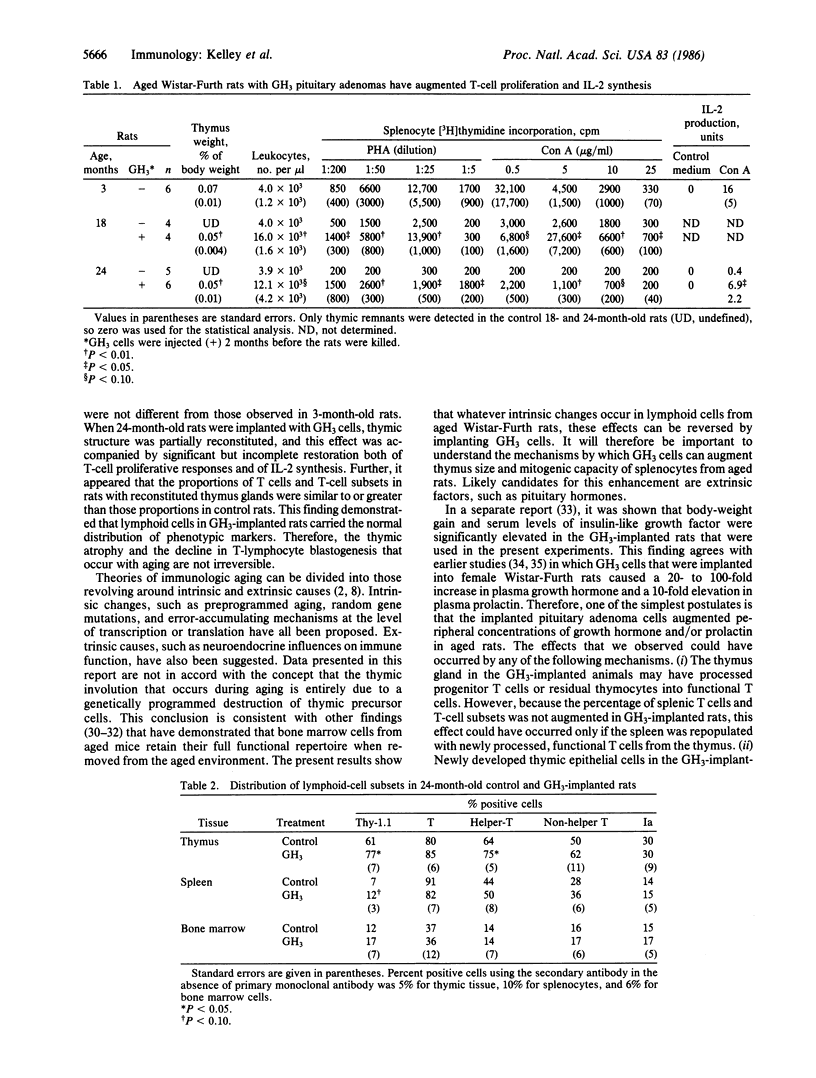
Abstract
Thymic size and T-cell function decrease with age, and it has not yet been possible to totally reverse this thymic atrophy and completely restore T-cell-dependent immune functions. In this study, GH3 pituitary adenoma cells, which secrete growth hormone and prolactin, were implanted subcutaneously into 16- and 22-month-old female Wistar-Furth rats and the rats were sacrificed approximately 2 months later. Only thymic remnants were detected in aged, non-implanted rats, but thymus glands were found in both the 18- and the 24-month-old rats that had been implanted with GH3 cells. Thymus glands from the GH3-implanted 18-month-old rats contained distinct cortical thymocytes and medullary epithelial cells. Depending on the concentration of phytohemagglutinin or concanavalin A, T-cell proliferative responses of splenocytes from these implanted rats were 2- to 5-fold greater than those of 18-month-old controls. At the optimal concentration of mitogen, proliferative responses to either lectin could be restored to those levels observed in splenocytes from 3-month-old Wistar-Furth females. Thymus glands from 24-month-old GH3-implanted rats contained more cortical thymocytes and fewer fat vacuoles than controls, but they were not totally reconstituted. No significant lectin-induced T-cell proliferative responses or IL-2 secretion were detected in 24-month-old control rats, but splenocytes from GH3-implanted rats showed augmented T-cell proliferative responses and increased synthesis of IL-2. Fluorescence-activated cell-sorter analysis of thymocytes revealed that 24-month-old rats implanted with GH3 cells had a higher proportion of lymphocytes with the Thy-1.1 and helper-T-cell phenotypes. These data show that it is possible to regenerate normal thymic tissue in situ and reverse the natural loss in cell-mediated immunity that occurs with aging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrenbrecht S. Specific binding of growth hormone to thymocytes. Nature. 1974 Nov 15;252(5480):255–257. doi: 10.1038/252255a0. [DOI] [PubMed] [Google Scholar]
- Astle C. M., Harrison D. E. Effects of marrow donor and recipient age on immune responses. J Immunol. 1984 Feb;132(2):673–677. [PubMed] [Google Scholar]
- Boockfor F. R., Hoeffler J. P., Frawley L. S. Cultures of GH3 cells are functionally heterogeneous: thyrotropin-releasing hormone, estradiol and cortisol cause reciprocal shifts in the proportions of growth hormone and prolactin secretors. Endocrinology. 1985 Jul;117(1):418–420. doi: 10.1210/endo-117-1-418. [DOI] [PubMed] [Google Scholar]
- Chang M. P., Makinodan T., Peterson W. J., Strehler B. L. Role of T cells and adherent cells in age-related decline in murine interleukin 2 production. J Immunol. 1982 Dec;129(6):2426–2430. [PubMed] [Google Scholar]
- Denckla W. D. Systems analysis of possible mechanisms of mammalian aging. Mech Ageing Dev. 1977 Mar-Apr;6(2):143–152. doi: 10.1016/0047-6374(77)90015-x. [DOI] [PubMed] [Google Scholar]
- Duquesnoy R. J. Immunodeficiency of the thymus-dependent system of the Ames dwarf mouse. J Immunol. 1972 Jun;108(6):1578–1590. [PubMed] [Google Scholar]
- Finkelstein J. W., Roffwarg H. P., Boyar R. M., Kream J., Hellman L. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab. 1972 Nov;35(5):665–670. doi: 10.1210/jcem-35-5-665. [DOI] [PubMed] [Google Scholar]
- Frasca D., Garavini M., Doria G. Recovery of T-cell functions in aged mice injected with synthetic thymosin-alpha 1. Cell Immunol. 1982 Sep 15;72(2):384–391. doi: 10.1016/0008-8749(82)90487-7. [DOI] [PubMed] [Google Scholar]
- Gil-Ad I., Gurewitz R., Marcovici O., Rosenfeld J., Laron Z. Effect of aging on human plasma growth hormone response to clonidine. Mech Ageing Dev. 1984 Sep;27(1):97–100. doi: 10.1016/0047-6374(84)90086-1. [DOI] [PubMed] [Google Scholar]
- Gillis S., Kozak R., Durante M., Weksler M. E. Immunological studies of aging. Decreased production of and response to T cell growth factor by lymphocytes from aged humans. J Clin Invest. 1981 Apr;67(4):937–942. doi: 10.1172/JCI110143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. C., Rosenberg J. S., Feldman J. D. T lymphocytes of young and aged rats. II. Functional defects and the role of interleukin-2. J Immunol. 1982 Feb;128(2):644–650. [PubMed] [Google Scholar]
- Gisler R. H., Schenkel-Hulliger L. Hormonal regulation of the immune response. II. Influence of pituitary and adrenal activity on immune responsiveness in vitro. Cell Immunol. 1971 Dec;2(6):646–657. doi: 10.1016/0008-8749(71)90012-8. [DOI] [PubMed] [Google Scholar]
- Goidl E. A., Choy J. W., Gibbons J. J., Weksler M. E., Thorbecke G. J., Siskind G. W. Production of auto-antiidiotypic antibody during the normal immune response. VII. Analysis of the cellular basis for the increased auto-antiidiotype antibody production by aged mice. J Exp Med. 1983 May 1;157(5):1635–1645. doi: 10.1084/jem.157.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynski R. M., Chang M. P., Kennedy M., MacRae S., Benzing K., Price G. B. Alterations in lymphocyte recognition repertoire during ageing. I. Analysis of changes in immune response potential of B lymphocytes from non-immunized aged mice, and the role of accessory cells in the expression of that potential. Immunopharmacology. 1984 Jun;7(3-4):179–194. doi: 10.1016/0162-3109(84)90035-3. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hirokawa K., Makinodan T. Thymic involution: effect on T cell differentiation. J Immunol. 1975 Jun;114(6):1659–1664. [PubMed] [Google Scholar]
- Kay M. M. Immunological aspects of aging: early changes in thymic activity. Mech Ageing Dev. 1984 Dec;28(2-3):193–218. doi: 10.1016/0047-6374(84)90020-4. [DOI] [PubMed] [Google Scholar]
- Kiess W., Butenandt O. Specific growth hormone receptors on human peripheral mononuclear cells: reexpression, identification, and characterization. J Clin Endocrinol Metab. 1985 Apr;60(4):740–746. doi: 10.1210/jcem-60-4-740. [DOI] [PubMed] [Google Scholar]
- Leclerc C., Morin A., Deriaud E., Chedid L. Inhibition of human IL 2 production by MDP and derivatives. J Immunol. 1984 Oct;133(4):1996–2000. [PubMed] [Google Scholar]
- Lesniak M. A., Gorden P., Roth J., Gavin J. R., 3rd Binding of 125I-human growth hormone to specific receptors in human cultured lymphocytes. Characterization of the interaction and a sensitive radioreceptor assay. J Biol Chem. 1974 Mar 25;249(6):1661–1667. [PubMed] [Google Scholar]
- Makinodan T., Kay M. M. Age influence on the immune system. Adv Immunol. 1980;29:287–330. doi: 10.1016/s0065-2776(08)60047-4. [DOI] [PubMed] [Google Scholar]
- Marguerite M. B. The thymus: clock for immunologic aging? J Invest Dermatol. 1979 Jul;73(1):29–38. doi: 10.1111/1523-1747.ep12532756. [DOI] [PubMed] [Google Scholar]
- Moloney W. C., Boschetti A. E., King V. Observations on leukemia in Wistar Furth rats. Cancer Res. 1969 Apr;29(4):938–946. [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I. Prolactin and contact sensitivity. Allergy. 1981 Aug;36(6):429–431. doi: 10.1111/j.1398-9995.1981.tb01850.x. [DOI] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Wren G. E., Asa S. L., Kovacs K. Immunomodulation by bromocriptine. Immunopharmacology. 1983 Oct;6(3):231–243. doi: 10.1016/0162-3109(83)90023-1. [DOI] [PubMed] [Google Scholar]
- Pandian M. R., Talwar G. P. Effect of growth hormone on the metabolism of thymus and on the immune response against sheep erythrocytes. J Exp Med. 1971 Nov 1;134(5):1095–1113. doi: 10.1084/jem.134.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W., Baroni C., Fabris N., Sorkin E. Hormones and immunological capacity. II. Reconstitution of antibody production in hormonally deficient mice by somatotropic hormone, thyrotropic hormone and thyroxin. Immunology. 1969 Feb;16(2):217–230. [PMC free article] [PubMed] [Google Scholar]
- Price G. B., Makinodan T. Immunologic deficiencies in senescence. II. Characterization of extrinsic deficiencies. J Immunol. 1972 Feb;108(2):413–417. [PubMed] [Google Scholar]
- Prysor-Jones R. A., Jenkins J. S. Effect of excessive secretion of growth hormone on tissues of the rat, with particular reference to the heart and skeletal muscle. J Endocrinol. 1980 Apr;85(1):75–82. doi: 10.1677/joe.0.0850075. [DOI] [PubMed] [Google Scholar]
- Rosenberg J. S., Gilman S. C., Feldman J. D. Effects of aging on cell cooperation and lymphocyte responsiveness to cytokines. J Immunol. 1983 Apr;130(4):1754–1758. [PubMed] [Google Scholar]
- Roth J. A., Kaeberle M. L., Grier R. L., Hopper J. G., Spiegel H. E., McAllister H. A. Improvement in clinical condition and thymus morphologic features associated with growth hormone treatment of immunodeficient dwarf dogs. Am J Vet Res. 1984 Jun;45(6):1151–1155. [PubMed] [Google Scholar]
- Russell D. H., Kibler R., Matrisian L., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human T and B lymphocytes: antagonism of prolactin binding by cyclosporine. J Immunol. 1985 May;134(5):3027–3031. [PubMed] [Google Scholar]
- Russell D. H., Matrisian L., Kibler R., Larson D. F., Poulos B., Magun B. E. Prolactin receptors on human lymphocytes and their modulation by cyclosporine. Biochem Biophys Res Commun. 1984 Jun 29;121(3):899–906. doi: 10.1016/0006-291x(84)90762-9. [DOI] [PubMed] [Google Scholar]
- SHREWSBURY M. M., REINHARDT W. O. Effect of pituitary growth hormone on lymphatic tissues, thoracic duct lymph flow, lymph protein and lymphocyte output in the rat. Endocrinology. 1959 Nov;65:858–860. doi: 10.1210/endo-65-5-858. [DOI] [PubMed] [Google Scholar]
- Seo H., Refetoff S., Fang V. S. Induction of hypothyroidism and hypoprolactinemia by growth hormone producing rat pituitary tumors. Endocrinology. 1977 Jan;100(1):216–226. doi: 10.1210/endo-100-1-216. [DOI] [PubMed] [Google Scholar]
- Snow E. C. Insulin and growth hormone function as minor growth factors that potentiate lymphocyte activation. J Immunol. 1985 Aug;135(2 Suppl):776s–778s. [PubMed] [Google Scholar]
- TAKEMOTO H., YOKORO K., FURTH J., COHEN A. I. Adrenotropic activity of mammo-somatotropic tumors in rats and mice. I. Biologic aspects. Cancer Res. 1962 Sep;22:917–924. [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Thoman M. L., Weigle W. O. Cell-mediated immunity in aged mice: an underlying lesion in IL 2 synthesis. J Immunol. 1982 May;128(5):2358–2361. [PubMed] [Google Scholar]
- Wade A. W., Szewczuk M. R. Aging, idiotype repertoire shifts, and compartmentalization of the mucosal-associated lymphoid system. Adv Immunol. 1984;36:143–188. doi: 10.1016/s0065-2776(08)60901-3. [DOI] [PubMed] [Google Scholar]
- Weksler M. C., Innes J. D., Goldstein G. Immunological studies of aging. IV. The contribution of thymic involution to the immune deficiencies of aging mice and reversal with thymopoietin32-36. J Exp Med. 1978 Oct 1;148(4):996–1006. doi: 10.1084/jem.148.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler M. E. The immune system and the aging process in man. Proc Soc Exp Biol Med. 1980 Nov;165(2):200–205. doi: 10.3181/00379727-165-40958. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Slanina S., Kado H., Melmed S. Autoregulation of pituitary growth hormone messenger ribonucleic acid levels in rats bearing transplantable mammosomatotrophic pituitary tumors. Endocrinology. 1986 Mar;118(3):915–918. doi: 10.1210/endo-118-3-915. [DOI] [PubMed] [Google Scholar]