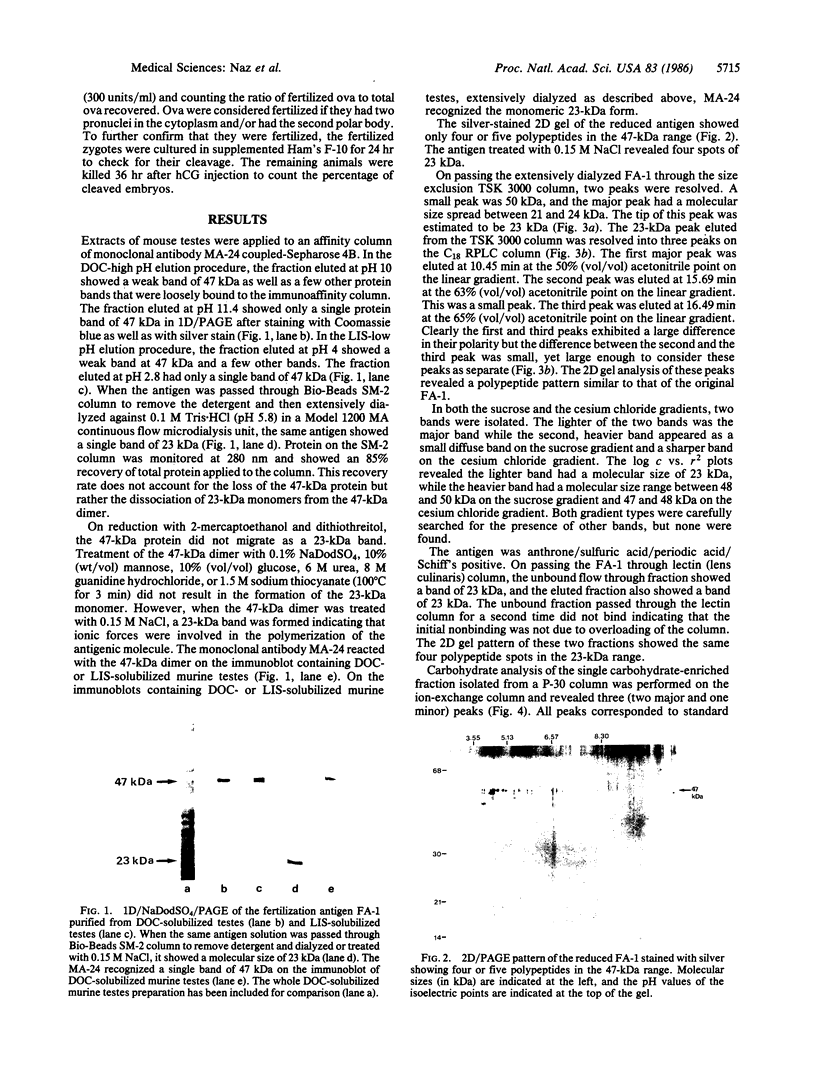
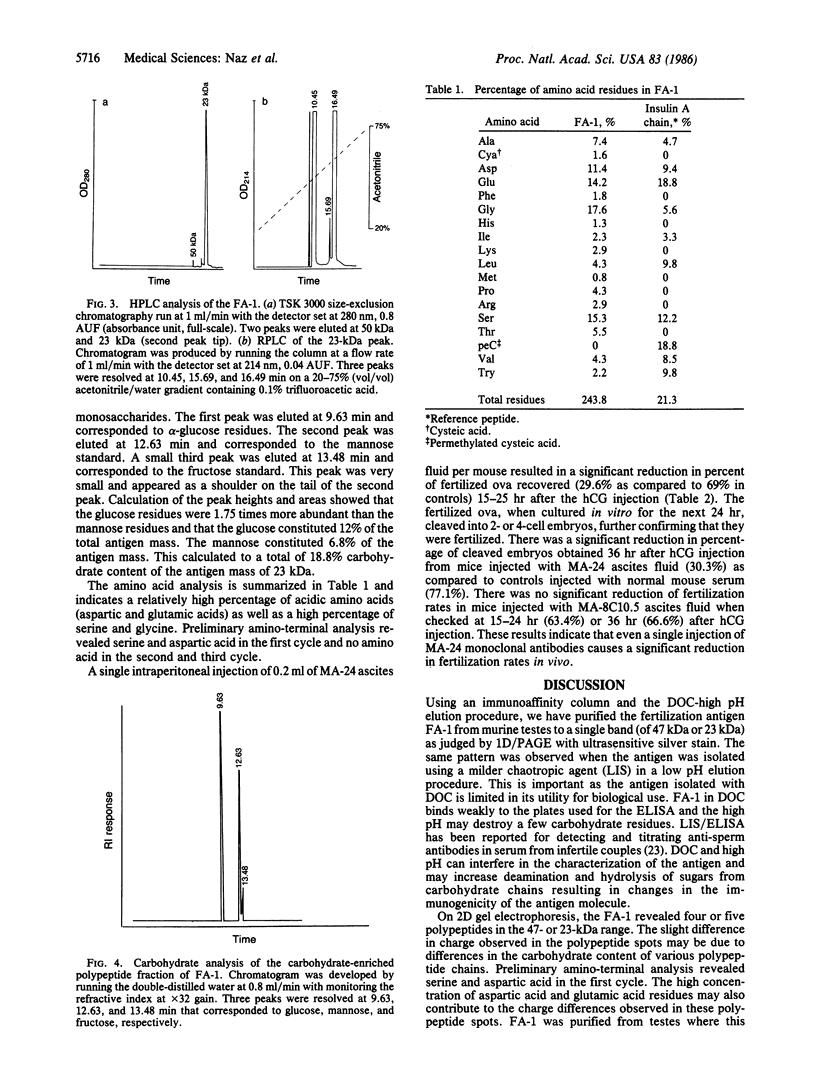
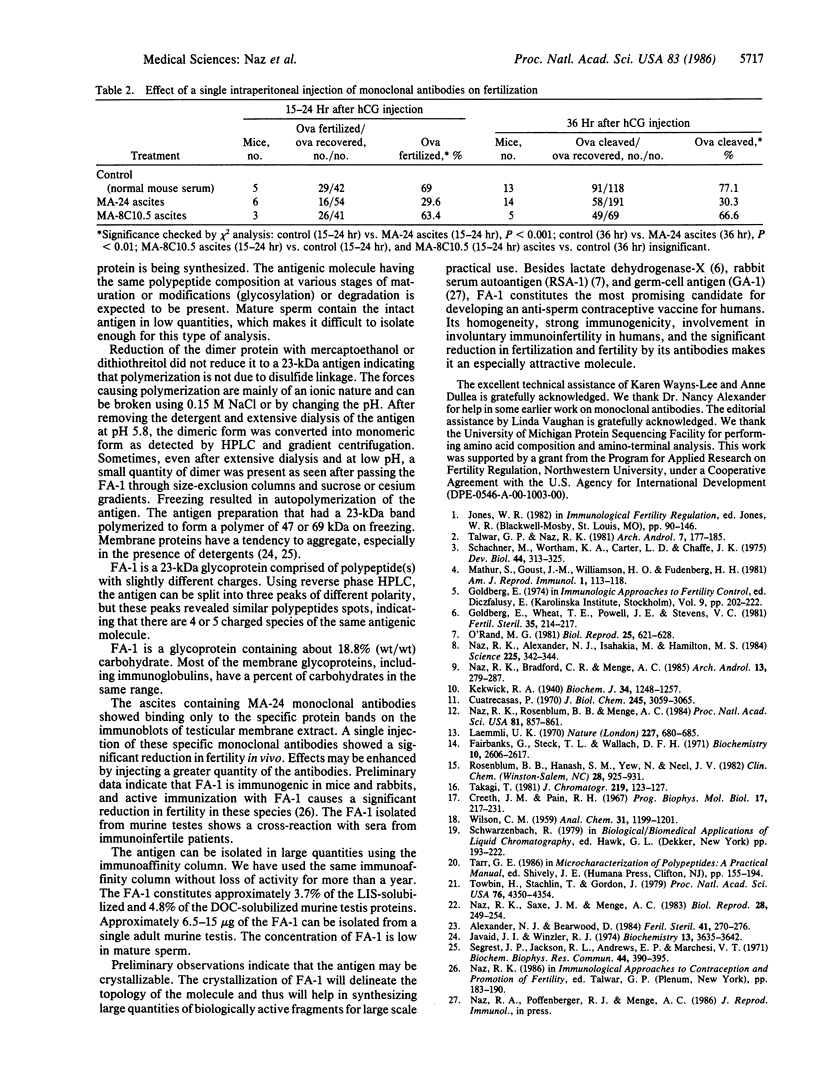
Abstract
A fertilization antigen, FA-1, was purified from either deoxycholate- or lithium diiodosalicylate-solubilized murine testes by immunoaffinity chromatography using a monoclonal antibody, MA-24, which inhibited fertilization in vitro. The FA-1 was recovered at high (11.4) or low (2.8) pH using stepwise elution procedures of the deoxycholate or lithium diiodosalicylate extracts, respectively. Both of these fractions showed a single band of 47 kDa when analyzed by NaDodSO4/PAGE and silver staining. Following removal of the detergent and extensive dialysis at pH 5.8 or treatment with 0.15 M NaCl, even in the presence of detergent, a monomer of 23 kDa was detected. Two-dimensional PAGE of FA-1 showed, four or five polypeptides in the 47-kDa or 23-kDa range. The dialyzed FA-1 contained a major 23-kDa and a minor 48-kDa band when separated on both sucrose and cesium chloride gradients. High performance size-exclusion chromatography showed a major peak at 23 kDa and a minor peak at 50 kDa. Further analysis of the 23-kDa peak by reverse-phase chromatography resolved the antigen into three peaks, which gave similar two-dimensional gel patterns as the native FA-1. Lectin affinity chromatography on a lens culinaris column demonstrated that a part of the antigen was bound to the lectin while the rest was not. The FA-1 revealed a positive reaction with periodic-Schiff reagent and contained glucose and mannose, which together constituted 18.8% of the total antigen mass. Amino acid analysis showed a high percentage of aspartic acid, glutamic acid, serine, and glycine. As a single injection of MA-24 significantly reduced fertilization rates in vivo, the purified FA-1 is an attractive candidate for the development of contraceptive vaccine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander N. J., Bearwood D. An immunosorption assay for antibodies to spermatozoa: comparison with agglutination and immobilization tests. Fertil Steril. 1984 Feb;41(2):270–276. doi: 10.1016/s0015-0282(16)47603-x. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Pain R. H. The determination of molecular weights of biological macromolecules by ultracentrifuge methods. Prog Biophys Mol Biol. 1967;17:217–287. doi: 10.1016/0079-6107(67)90008-9. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Goldberg E., Wheat T. E., Powell J. E., Stevens V. C. Reduction of fertility in female baboons immunized with lactate dehydrogenase C4. Fertil Steril. 1981 Feb;35(2):214–217. doi: 10.1016/s0015-0282(16)45325-2. [DOI] [PubMed] [Google Scholar]
- Javaid J. I., Winzler R. J. Association of glycoproteins with the membranes. II. Isolation and partial characterization of "lipophilic fragment" from human erythrocyte membrane glycoprotein. Biochemistry. 1974 Aug 27;13(18):3639–3642. doi: 10.1021/bi00715a002. [DOI] [PubMed] [Google Scholar]
- Kekwick R. A. The serum proteins in multiple myelomatosis. Biochem J. 1940 Sep;34(8-9):1248–1257. doi: 10.1042/bj0341248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mathur S., Goust J. M., Williamson H. O., Fudenberg H. H. Cross-reactivity of sperm and T lymphocyte antigens. Am J Reprod Immunol. 1981;1(3):113–118. doi: 10.1111/j.1600-0897.1981.tb00142.x. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Alexander N. J., Isahakia M., Hamilton M. S. Monoclonal antibody to a human germ cell membrane glycoprotein that inhibits fertilization. Science. 1984 Jul 20;225(4659):342–344. doi: 10.1126/science.6539947. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Bradford C. R., Menge A. C. Isoantigenicity of rabbit sperm, testis, and their extracts as demonstrated by Western blot enzyme immunobinding procedure. Arch Androl. 1984;13(2-3):279–287. doi: 10.3109/01485018408987528. [DOI] [PubMed] [Google Scholar]
- Naz R. K., Rosenblum B. B., Menge A. C. Characterization of a membrane antigen from rabbit testis and sperm isolated by using monoclonal antibodies and effect of its antiserum on fertility. Proc Natl Acad Sci U S A. 1984 Feb;81(3):857–861. doi: 10.1073/pnas.81.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz R. K., Saxe J. M., Menge A. C. Inhibition of fertility in rabbits by monoclonal antibodies against sperm. Biol Reprod. 1983 Feb;28(1):249–254. doi: 10.1095/biolreprod28.1.249. [DOI] [PubMed] [Google Scholar]
- O'Rand M. G. Inhibition of fertility and sperm-zona binding by antiserum to the rabbit sperm membrane autoantigen RSA-1. Biol Reprod. 1981 Oct;25(3):621–628. doi: 10.1095/biolreprod25.3.621. [DOI] [PubMed] [Google Scholar]
- Rosenblum B. B., Hanash S. M., Yew N., Neel J. V. Two-dimensional electrophoretic analysis of erythrocyte membranes. Clin Chem. 1982 Apr;28(4 Pt 2):925–931. [PubMed] [Google Scholar]
- Schachner M., Wortham K. A., Carter L. D., Chaffee J. K. NS-4 (nervous system antigen-4), a cell surface antigen of developing and adult mouse brain and sperm. Dev Biol. 1975 Jun;44(2):313–325. doi: 10.1016/0012-1606(75)90402-9. [DOI] [PubMed] [Google Scholar]
- Segrest J. P., Jackson R. L., Andrews E. P., Marchesi V. T. Human erythrocyte membrane glycoprotein: a re-evaluation of the molecular weight as determined by SDS polyacrylamide gel electrophoresis. Biochem Biophys Res Commun. 1971 Jul 16;44(2):390–395. doi: 10.1016/0006-291x(71)90612-7. [DOI] [PubMed] [Google Scholar]
- Talwar G. P., Naz R. K. Immunological control of male fertility. Arch Androl. 1981 Sep;7(2):177–185. doi: 10.3109/01485018108999305. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]