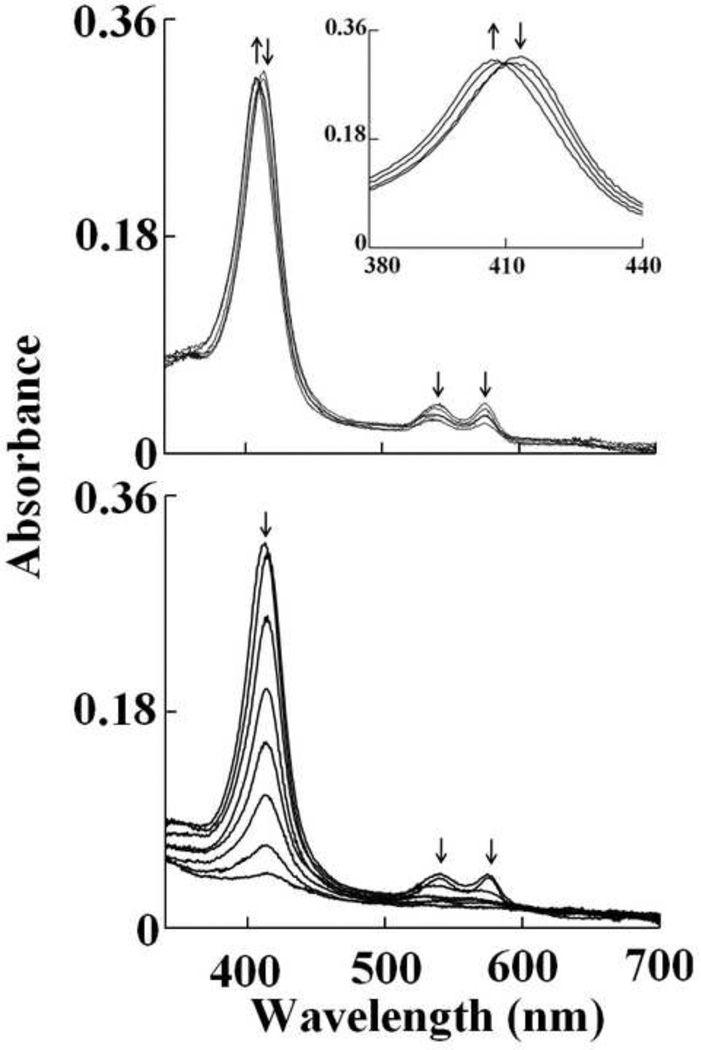
Fig. 3.
Oxy-hemoglobin heme oxidation and subsequent heme destruction mediated by HOCl. Absorbance spectra recorded by diode array stopped-flow when a phosphate buffer solution (0.20 M, pH 7.0) containing 1.25 µM oxy-Hb was rapidly mixed with a buffer solution containing increasing concentration of HOCl, at 10°C. The upper panel shows heme oxidation when the protein solution was rapidly mixed with a buffer solution supplemented with 20 µM, HOCl, spectra were collected after 0.5, 1.5, 24.5 and 599.5 s of initiating the reaction. The inset shows the transition of the Soret absorbance peak from 415 nm (oxy-Hb) to 405 nm (met-Hb). The lower panel shows Hb heme destruction when the oxy-Hb solution was rapidly mixed with 400 µM HOCl, spectra were collected after 0.5, 1.5, 6.5, 19.5, 44.5, 89.5, 199.5, 599.5 s of initiating the reaction. Arrows indicate the direction of spectral change over time. The data are representative of three independent experiments.