Figure 2.
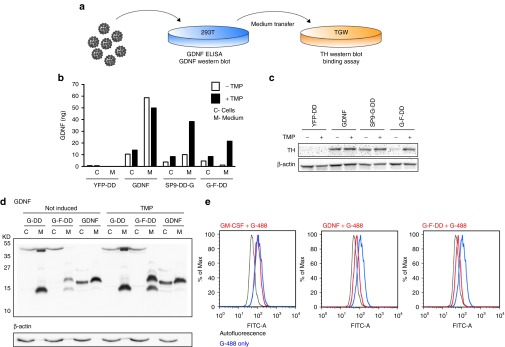
In vitro screening of second-generation DD. (a) To screen for regulation, activity, processing, and binding of second-generation DD, lentiviral vectors were used to transduce 293T cells and the medium was subsequently transferred to TGW cells. (b) GDNF ELISA of medium (M) or intracellular protein (C) from 293T cells transduced at an multiplicity of infection of 2.5 with lentiviral vectors expressing N-terminal fusion of DD to yellow fluorescence protein (YFP-DD), GDNF, SP9-DD-G, or G-F-DD. Five days after transduction, the cells were stimulated with 1 × 10−5 mol/l TMP for 24 hours. (c) Culture media from transduced 293T was transferred to TGW cells and 24 hours after the cells were processed for western blot and probed for TH, using β-actin as loading control. (d) Medium (M) and cells (C) from 293T cells transduced with lentiviral vectors transduced with lentiviral vectors expressing GDNF, G-DD, or G-F-DD and treated with TMP as described above was used for western blot and probed for GDNF. (e) Medium containing 1 × 10−8 mol/l granulocyte monocyte colony stimulating factor (GM-CSF), GDNF or G-F-DD was added to TGW cells together with 2 × 10−8 mol/l GDNF labeled with alexa 488 (G-488). Four hours after the proteins were added, the cells were analyzed by FACS. DD, destabilizing domains; GDNF, glial cell line–derived neurotrophic factor; YFP, yellow fluorescence protein.
