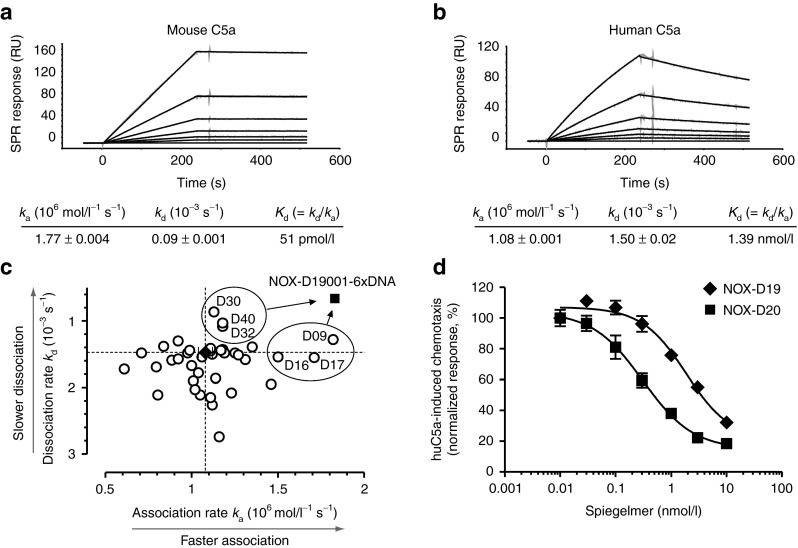
Figure 2.
Characterization and postselection optimization of C5a-binding Spiegelmer. SPR measurement of NOX-D19 binding to (a) mouse and (b) human C5a. Kinetic rate constants ka and kd are shown as mean ± SEM. Data are representative for at least 3 individual measurements. (c) Kinetic rate constants of 2′-deoxyribonucleotide-modified NOX-D19001 variants binding to huC5a were determined by SPR measurement. For unmodified NOX-D19001 (black diamond; dotted lines) mean ± SD of 5 injections is shown. Six modified variants of NOX-D19001 (D09, D16, D17, D30, D32, and D40) with increased overall affinity (Kd = kd/ka) were chosen and combined to the six-times modified Spiegelmer NOX-D19001-6xDNA (black square). (d) Inhibition by NOX-D19 (black diamonds) and NOX-D20 (black squares) of CD88+ BA/F3 cell chemotaxis stimulated with 0.1 nmol/l huC5a. Mean ± SD of triplicate measurements is shown. Data are representative for four independent experiments. RU, response units.