Figure 5.
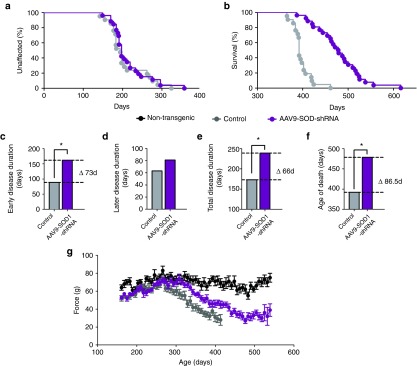
AAV9-SOD1-shRNA improves survival and motor performance in SOD1G37R mice treated after disease onset. (a) There was no difference in median disease onset between AAV9-SOD1-shRNA– and control-treated mice (average age at treatment = 215 days versus median onset of 194 days control and 197 days treated; log-rank test, P = 0.46). (b,f) Median survival of AAV9-SOD1-shRNA–treated SOD1G37R mice (n = 25) was significantly extended versus control mice (n = 21) (control, n = 21, 392 days; SOD1 shRNA, n = 25, 478.5 days; log-rank test, P < 0.0001). (c–e) The early phase of disease was significantly slowed by 73 days in treated mice as compared with control mice (control: 89 days; SOD1 shRNA: 162 days; P < 0.0001; Wilcoxon signed-rank test) while the late phase of disease showed a nonsignificant slowing (control: 63 days; SOD1 shRNA: 81 days; P = 0.14, Wilcoxon signed-rank test). Together this amounted to a 66-day increase in median disease duration (control: 173 days; SOD1 shRNA: 239 days; P < 0.0001; Wilcoxon signed-rank test). (g) A trend to improved hindlimb grip strength appeared in AAV9-SOD1-shRNA–treated mice compared with control mice.
