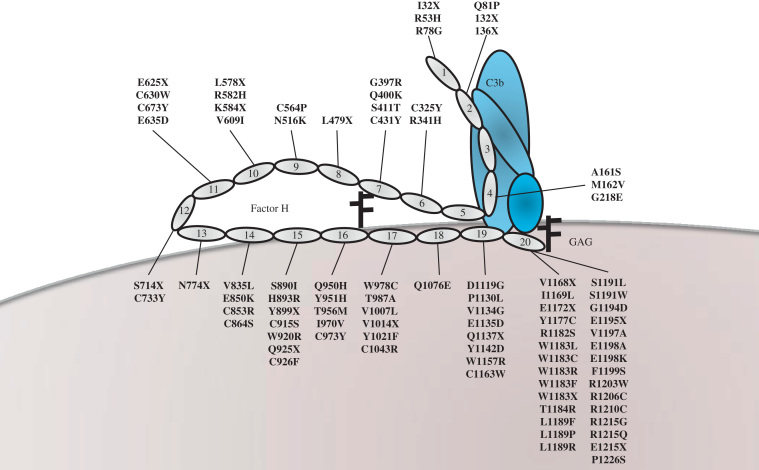
Figure 2.
CFH and aHUS-associated mutations. CFH is composed of 20 CCP modules. The N-terminal modules (CCP1-4) bind to C3b and act as a cofactor for the CFI-mediated cleavage to the inactive iC3b. The C-terminal modules (CCP19 and 20) bind to C3b and glycosaminoglycans on host cells to mediate cell surface protection. Genetic variants described in aHUS cluster in CCPs 19 and 20, but can be seen throughout the molecule. Functional analysis of aHUS-associated variants has focused predominantly on the C-terminal variants (Table 3).