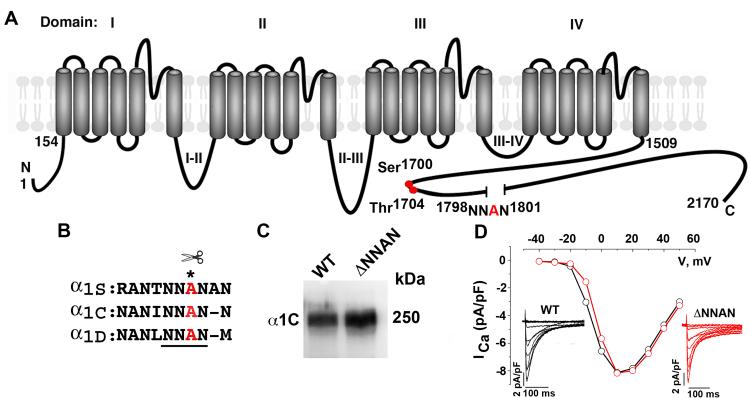
Figure 1. Deletion of proteolytic cleavage site does not affect heterologously expressed CaV1.2 channel expression or function.
(A) Schematic of cardiac α1C subunit topology. The putative proteolytic cleavage site, 1798NNAN1801 is identified. Red circles are putative PKA (Ser1700) and casein kinase II (Thr1704) phosphorylation sites. (B) Highly conserved amino acid sequences surrounding putative proteolytic cleavage site, marked by asterisk. (C) Anti-α1C antibody immunoblot of extracts from WT α1C and ΔNNAN α1C expressing tsA-201 cells. (D) WT (black) and ΔNNAN α1C (red) current-voltage relationships and current traces (inset). Currents elicited by 400-ms test pulses between −60 mV to +60 mV from a holding potential of −70 mV.