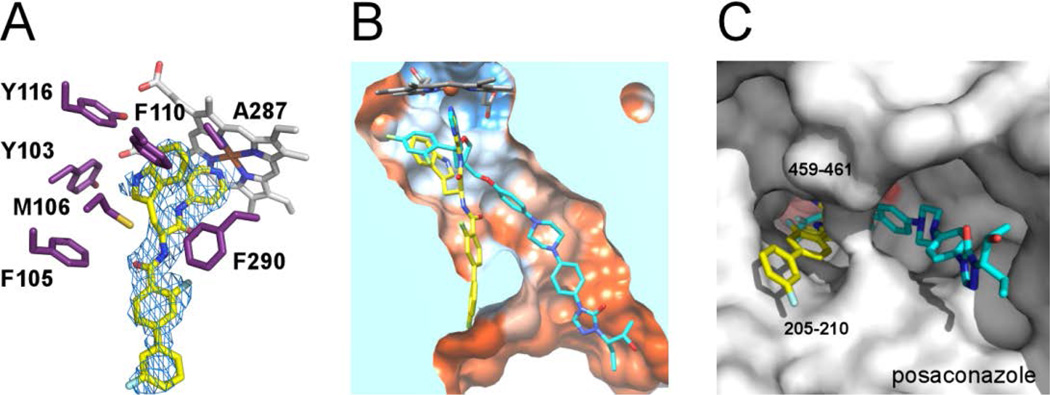
Figure 2. X-ray co-crystal structure of the Tb CYP51-14t complex.
(A) Electron density map (blue mesh) contoured at 1.0 σ delineates the positions of 14t (yellow sticks) in the active site. In purple are amino acid residues providing hydrophobic contacts within 5 Å to the indol moiety of 14t plus F105. Heme is displayed as grey sticks. (B) View of the 14t-bound CYP51 clipped by a plane through the binding site compares the binding modes of 14t (yellow) and posaconazole (cyan). The structure of TbCYP51 complexed with posaconazole (PDB code: 2×2N) is superimposed on that of with 14t. The active site surface is colored by hydrophobicity from orange (lipophilic) to blue (hydrophylic). (C) View of bound inhibitors from the entrance to the active site. The enzyme is represented by a gray surface. The hydrophobic units of posaconazole and 14t occupy different hydrophobic tunnels in corresponding co-crystal structures. The images here and otherwise were generated using PYMOL29 or CHIMERA30.