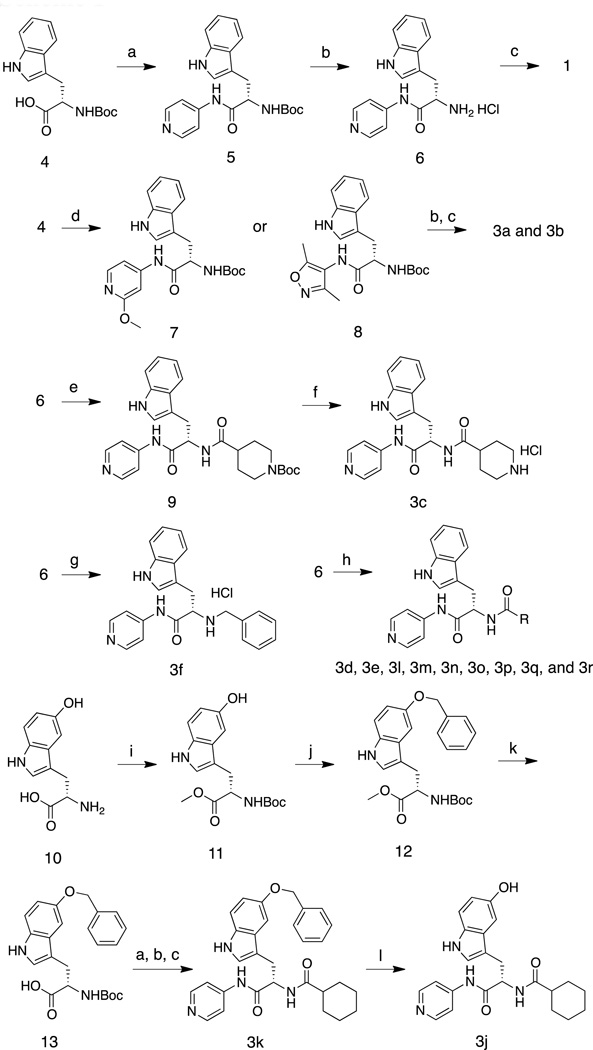
Scheme 1.
Reagents and conditions: (a) PyBOP, HOBt, NEt3, CH2Cl2, 4-aminopyridine, 0 °C to room temp., 1h, 94%. (b) 4N HCl in dioxane, dioxane, room temp., 12h, >90% (crude) (c) cyclohexancarbonyl chloride, NEt3, CH2Cl2, 0 °C to room temp., 1h, 94%. (d) PyBOP, HOBt, NEt3, CH2Cl2, 4-amino-2-methoxypyridine or 4-amino-3,5-dimethylisoxazole, 0 °C to room temp., 1h, 84%. (e) pentafluorophenyl trifluoroacetate, 1-Boc-isonipecotic acid, NEt3, CH2Cl2, 0 °C to room temp., 1h, 53%. (f) trifluoroacetic acid, CH2Cl2, room temp., 1h, 47%. (g) benzylbromide, NEt3, CH2Cl2, room temp., 12h, 30%. (h) pentafluorophenyl trifluoroacetate, NEt3, CH2Cl2, alkyl carboxylic acids, 0 °C to room temperature, 1h, ~80%. (i) SOCl2, CH3OH, 0 °C to room temp., 12h, then (Boc)2O, NEt3, CH2Cl2, 0 °C to room temp., 6h, 82%. (j) benzylbromide, Cs2CO3, acetone, room temp., 12h, 77%. (k) 10% NaOH, CH3OH, 0 °C, 2h, 97%. (l) H2, Pd/C, CH3OH/THF, room temp., 24h, 29%.