Abstract
As an essential nutrient and a potential toxin, iron poses an exquisite regulatory problem in biology and medicine. At the cellular level, the basic molecular framework for the regulation of iron uptake, storage, and utilization has been defined. Two cytoplasmic RNA-binding proteins, iron-regulatory protein-1 (IRP-1) and IRP-2, respond to changes in cellular iron availability and coordinate the expression of mRNAs that harbor IRP-binding sites, iron-responsive elements (IREs). Nitric oxide (NO) and oxidative stress in the form of H2O2 also signal to IRPs and thereby influence cellular iron metabolism. The recent discovery of two IRE-regulated mRNAs encoding enzymes of the mitochondrial citric acid cycle may represent the beginnings of elucidating regulatory coupling between iron and energy metabolism. In addition to providing insights into the regulation of iron metabolism and its connections with other cellular pathways, the IRE/IRP system has emerged as a prime example for the understanding of translational regulation and mRNA stability control. Finally, IRP-1 has highlighted an unexpected role for iron sulfur clusters as post-translational regulatory switches.
Full text
PDF


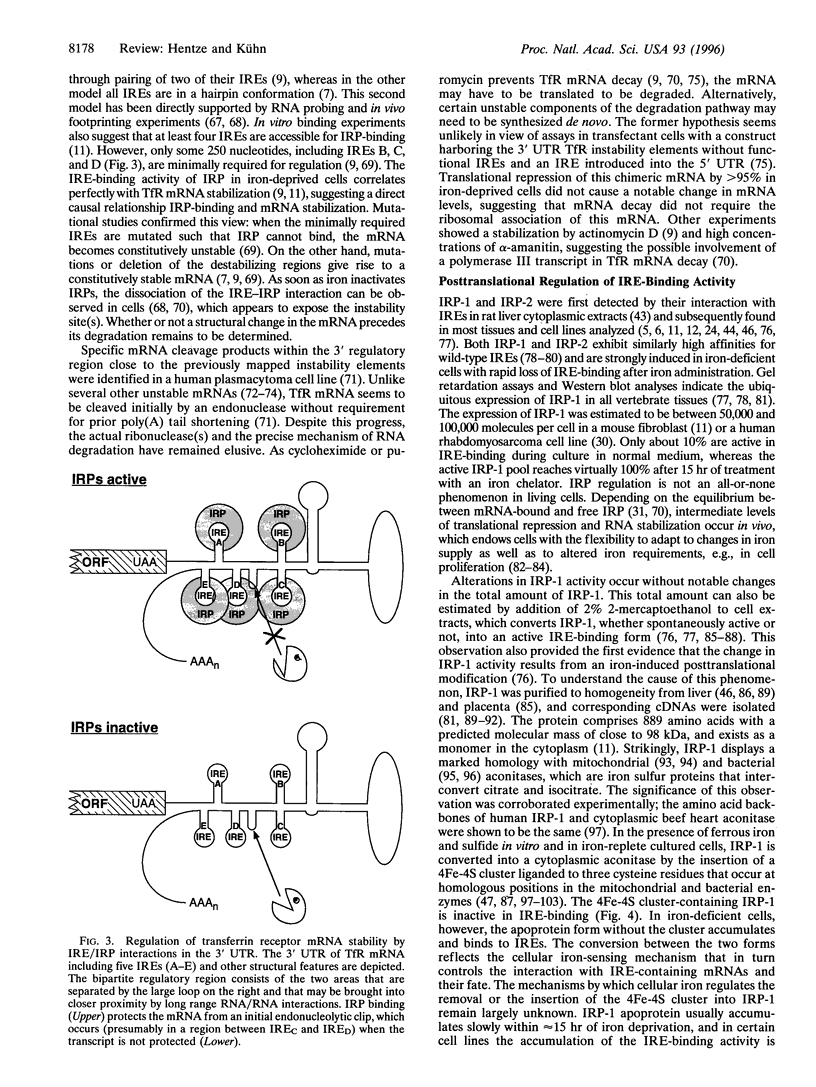




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aziz N., Munro H. N. Iron regulates ferritin mRNA translation through a segment of its 5' untranslated region. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8478–8482. doi: 10.1073/pnas.84.23.8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balla J., Jacob H. S., Balla G., Nath K., Eaton J. W., Vercellotti G. M. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton H. A., Eisenstein R. S., Bomford A., Munro H. N. Determinants of the interaction between the iron-responsive element-binding protein and its binding site in rat L-ferritin mRNA. J Biol Chem. 1990 Apr 25;265(12):7000–7008. [PubMed] [Google Scholar]
- Basilion J. P., Kennedy M. C., Beinert H., Massinople C. M., Klausner R. D., Rouault T. A. Overexpression of iron-responsive element-binding protein and its analytical characterization as the RNA-binding form, devoid of an iron-sulfur cluster. Arch Biochem Biophys. 1994 Jun;311(2):517–522. doi: 10.1006/abbi.1994.1270. [DOI] [PubMed] [Google Scholar]
- Basilion J. P., Rouault T. A., Massinople C. M., Klausner R. D., Burgess W. H. The iron-responsive element-binding protein: localization of the RNA-binding site to the aconitase active-site cleft. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):574–578. doi: 10.1073/pnas.91.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont C., Leneuve P., Devaux I., Scoazec J. Y., Berthier M., Loiseau M. N., Grandchamp B., Bonneau D. Mutation in the iron responsive element of the L ferritin mRNA in a family with dominant hyperferritinaemia and cataract. Nat Genet. 1995 Dec;11(4):444–446. doi: 10.1038/ng1295-444. [DOI] [PubMed] [Google Scholar]
- Bertrand E., Fromont-Racine M., Pictet R., Grange T. Visualization of the interaction of a regulatory protein with RNA in vivo. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3496–3500. doi: 10.1073/pnas.90.8.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettany A. J., Eisenstein R. S., Munro H. N. Mutagenesis of the iron-regulatory element further defines a role for RNA secondary structure in the regulation of ferritin and transferrin receptor expression. J Biol Chem. 1992 Aug 15;267(23):16531–16537. [PubMed] [Google Scholar]
- Bhasker C. R., Burgiel G., Neupert B., Emery-Goodman A., Kühn L. C., May B. K. The putative iron-responsive element in the human erythroid 5-aminolevulinate synthase mRNA mediates translational control. J Biol Chem. 1993 Jun 15;268(17):12699–12705. [PubMed] [Google Scholar]
- Binder R., Horowitz J. A., Basilion J. P., Koeller D. M., Klausner R. D., Harford J. B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3' UTR and does not involve poly(A) tail shortening. EMBO J. 1994 Apr 15;13(8):1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. H., Daniels-McQueen S., Walden W. E., Patino M. M., Gaffield L., Bielser D., Thach R. E. Requirements for the translational repression of ferritin transcripts in wheat germ extracts by a 90-kDa protein from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13383–13386. [PubMed] [Google Scholar]
- Cairo G., Tacchini L., Pogliaghi G., Anzon E., Tomasi A., Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the "free" iron pool. J Biol Chem. 1995 Jan 13;270(2):700–703. doi: 10.1074/jbc.270.2.700. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K., Klausner R. D., Harford J. B. Two genetic loci participate in the regulation by iron of the gene for the human transferrin receptor. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1787–1791. doi: 10.1073/pnas.85.6.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Hentze M. W., Koeller D. M., Caughman S. W., Rouault T. A., Klausner R. D., Harford J. B. Iron-responsive elements: regulatory RNA sequences that control mRNA levels and translation. Science. 1988 May 13;240(4854):924–928. doi: 10.1126/science.2452485. [DOI] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caughman S. W., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. The iron-responsive element is the single element responsible for iron-dependent translational regulation of ferritin biosynthesis. Evidence for function as the binding site for a translational repressor. J Biol Chem. 1988 Dec 15;263(35):19048–19052. [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chan L. N., Grammatikakis N., Banks J. M., Gerhardt E. M. Chicken transferrin receptor gene: conservation 3' noncoding sequences and expression in erythroid cells. Nucleic Acids Res. 1989 May 25;17(10):3763–3771. doi: 10.1093/nar/17.10.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. Y., Shyu A. B. AU-rich elements: characterization and importance in mRNA degradation. Trends Biochem Sci. 1995 Nov;20(11):465–470. doi: 10.1016/s0968-0004(00)89102-1. [DOI] [PubMed] [Google Scholar]
- Constable A., Quick S., Gray N. K., Hentze M. W. Modulation of the RNA-binding activity of a regulatory protein by iron in vitro: switching between enzymatic and genetic function? Proc Natl Acad Sci U S A. 1992 May 15;89(10):4554–4558. doi: 10.1073/pnas.89.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson R. M., Cleveland D. W. Ferritin synthesis is controlled by iron-dependent translational derepression and by changes in synthesis/transport of nuclear ferritin RNAs. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7613–7617. doi: 10.1073/pnas.90.16.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L. A., Adrian G. S. Posttranscriptional regulation of chimeric human transferrin genes by iron. Biochemistry. 1993 May 11;32(18):4738–4745. doi: 10.1021/bi00069a007. [DOI] [PubMed] [Google Scholar]
- Cox L. A., Kennedy M. C., Adrian G. S. The 5'-untranslated region of human transferrin mRNA, which contains a putative iron-regulatory element, is bound by purified iron-regulatory protein in a sequence-specific manner. Biochem Biophys Res Commun. 1995 Jul 26;212(3):925–932. doi: 10.1006/bbrc.1995.2058. [DOI] [PubMed] [Google Scholar]
- Cox T. C., Bawden M. J., Martin A., May B. K. Human erythroid 5-aminolevulinate synthase: promoter analysis and identification of an iron-responsive element in the mRNA. EMBO J. 1991 Jul;10(7):1891–1902. doi: 10.1002/j.1460-2075.1991.tb07715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandekar T., Hentze M. W. Finding the hairpin in the haystack: searching for RNA motifs. Trends Genet. 1995 Feb;11(2):45–50. doi: 10.1016/s0168-9525(00)88996-9. [DOI] [PubMed] [Google Scholar]
- Dandekar T., Stripecke R., Gray N. K., Goossen B., Constable A., Johansson H. E., Hentze M. W. Identification of a novel iron-responsive element in murine and human erythroid delta-aminolevulinic acid synthase mRNA. EMBO J. 1991 Jul;10(7):1903–1909. doi: 10.1002/j.1460-2075.1991.tb07716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRusso P. A., Philpott C. C., Iwai K., Mostowski H. S., Klausner R. D., Rouault T. A. Expression of a constitutive mutant of iron regulatory protein 1 abolishes iron homeostasis in mammalian cells. J Biol Chem. 1995 Jun 30;270(26):15451–15454. doi: 10.1074/jbc.270.26.15451. [DOI] [PubMed] [Google Scholar]
- Decker C. J., Parker R. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes Dev. 1993 Aug;7(8):1632–1643. doi: 10.1101/gad.7.8.1632. [DOI] [PubMed] [Google Scholar]
- Dickey L. F., Wang Y. H., Shull G. E., Wortman I. A., 3rd, Theil E. C. The importance of the 3'-untranslated region in the translational control of ferritin mRNA. J Biol Chem. 1988 Mar 5;263(7):3071–3074. [PubMed] [Google Scholar]
- Dix D. J., Lin P. N., McKenzie A. R., Walden W. E., Theil E. C. The influence of the base-paired flanking region on structure and function of the ferritin mRNA iron regulatory element. J Mol Biol. 1993 May 20;231(2):230–240. doi: 10.1006/jmbi.1993.1278. [DOI] [PubMed] [Google Scholar]
- Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993 Sep;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale J. W., Munro H. N. Regulation of synthesis and turnover of ferritin in rat liver. J Biol Chem. 1966 Aug 10;241(15):3630–3637. [PubMed] [Google Scholar]
- Dunkov B. C., Zhang D., Choumarov K., Winzerling J. J., Law J. H. Isolation and characterization of mosquito ferritin and cloning of a cDNA that encodes one subunit. Arch Insect Biochem Physiol. 1995;29(3):293–307. doi: 10.1002/arch.940290307. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Tuazon P. T., Schalinske K. L., Anderson S. A., Traugh J. A. Iron-responsive element-binding protein. Phosphorylation by protein kinase C. J Biol Chem. 1993 Dec 25;268(36):27363–27370. [PubMed] [Google Scholar]
- Emery-Goodman A., Hirling H., Scarpellino L., Henderson B., Kühn L. C. Iron regulatory factor expressed from recombinant baculovirus: conversion between the RNA-binding apoprotein and Fe-S cluster containing aconitase. Nucleic Acids Res. 1993 Mar 25;21(6):1457–1461. doi: 10.1093/nar/21.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessling L. S., Daniels-McQueen S., Bhattacharyya-Pakrasi M., Lin J. J., Thach R. E. Enhanced degradation of the ferritin repressor protein during induction of ferritin messenger RNA translation. Science. 1992 May 1;256(5057):670–673. doi: 10.1126/science.1316633. [DOI] [PubMed] [Google Scholar]
- Goossen B., Caughman S. W., Harford J. B., Klausner R. D., Hentze M. W. Translational repression by a complex between the iron-responsive element of ferritin mRNA and its specific cytoplasmic binding protein is position-dependent in vivo. EMBO J. 1990 Dec;9(12):4127–4133. doi: 10.1002/j.1460-2075.1990.tb07635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossen B., Hentze M. W. Position is the critical determinant for function of iron-responsive elements as translational regulators. Mol Cell Biol. 1992 May;12(5):1959–1966. doi: 10.1128/mcb.12.5.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Hentze M. W. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994 Aug 15;13(16):3882–3891. doi: 10.1002/j.1460-2075.1994.tb06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Hentze M. W. Regulation of protein synthesis by mRNA structure. Mol Biol Rep. 1994 May;19(3):195–200. doi: 10.1007/BF00986961. [DOI] [PubMed] [Google Scholar]
- Gray N. K., Pantopoulos K., Dandekar T., Ackrell B. A., Hentze M. W. Translational regulation of mammalian and Drosophila citric acid cycle enzymes via iron-responsive elements. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4925–4930. doi: 10.1073/pnas.93.10.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Quick S., Goossen B., Constable A., Hirling H., Kühn L. C., Hentze M. W. Recombinant iron-regulatory factor functions as an iron-responsive-element-binding protein, a translational repressor and an aconitase. A functional assay for translational repression and direct demonstration of the iron switch. Eur J Biochem. 1993 Dec 1;218(2):657–667. doi: 10.1111/j.1432-1033.1993.tb18420.x. [DOI] [PubMed] [Google Scholar]
- Gruer M. J., Guest J. R. Two genetically-distinct and differentially-regulated aconitases (AcnA and AcnB) in Escherichia coli. Microbiology. 1994 Oct;140(Pt 10):2531–2541. doi: 10.1099/00221287-140-10-2531. [DOI] [PubMed] [Google Scholar]
- Guo B., Brown F. M., Phillips J. D., Yu Y., Leibold E. A. Characterization and expression of iron regulatory protein 2 (IRP2). Presence of multiple IRP2 transcripts regulated by intracellular iron levels. J Biol Chem. 1995 Jul 14;270(28):16529–16535. doi: 10.1074/jbc.270.28.16529. [DOI] [PubMed] [Google Scholar]
- Guo B., Phillips J. D., Yu Y., Leibold E. A. Iron regulates the intracellular degradation of iron regulatory protein 2 by the proteasome. J Biol Chem. 1995 Sep 15;270(37):21645–21651. doi: 10.1074/jbc.270.37.21645. [DOI] [PubMed] [Google Scholar]
- Guo B., Yu Y., Leibold E. A. Iron regulates cytoplasmic levels of a novel iron-responsive element-binding protein without aconitase activity. J Biol Chem. 1994 Sep 30;269(39):24252–24260. [PubMed] [Google Scholar]
- Haile D. J., Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Regulation of interaction of the iron-responsive element binding protein with iron-responsive RNA elements. Mol Cell Biol. 1989 Nov;9(11):5055–5061. doi: 10.1128/mcb.9.11.5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Klausner R. D. The inhibition of the iron responsive element RNA-protein interaction by heme does not mimic in vivo iron regulation. J Biol Chem. 1990 Aug 5;265(22):12786–12789. [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7536–7540. doi: 10.1073/pnas.89.16.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausladen A., Fridovich I. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J Biol Chem. 1994 Nov 25;269(47):29405–29408. [PubMed] [Google Scholar]
- Henderson B. R., Kühn L. C. Differential modulation of the RNA-binding proteins IRP-1 and IRP-2 in response to iron. IRP-2 inactivation requires translation of another protein. J Biol Chem. 1995 Sep 1;270(35):20509–20515. doi: 10.1074/jbc.270.35.20509. [DOI] [PubMed] [Google Scholar]
- Henderson B. R., Menotti E., Bonnard C., Kühn L. C. Optimal sequence and structure of iron-responsive elements. Selection of RNA stem-loops with high affinity for iron regulatory factor. J Biol Chem. 1994 Jul 1;269(26):17481–17489. [PubMed] [Google Scholar]
- Henderson B. R., Menotti E., Kühn L. C. Iron regulatory proteins 1 and 2 bind distinct sets of RNA target sequences. J Biol Chem. 1996 Mar 1;271(9):4900–4908. doi: 10.1074/jbc.271.9.4900. [DOI] [PubMed] [Google Scholar]
- Henderson B. R., Seiser C., Kühn L. C. Characterization of a second RNA-binding protein in rodents with specificity for iron-responsive elements. J Biol Chem. 1993 Dec 25;268(36):27327–27334. [PubMed] [Google Scholar]
- Henry Y., Lepoivre M., Drapier J. C., Ducrocq C., Boucher J. L., Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993 Sep;7(12):1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Casey J. L., Koeller D. M., Rouault T. A., Harford J. B., Klausner R. D. A model for the structure and functions of iron-responsive elements. Gene. 1988 Dec 10;72(1-2):201–208. doi: 10.1016/0378-1119(88)90145-x. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Caughman S. W., Rouault T. A., Barriocanal J. G., Dancis A., Harford J. B., Klausner R. D. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987 Dec 11;238(4833):1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Caughman S. W., Dancis A., Harford J. B., Klausner R. D. A cis-acting element is necessary and sufficient for translational regulation of human ferritin expression in response to iron. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6730–6734. doi: 10.1073/pnas.84.19.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Hershko C. Control of disease by selective iron depletion: a novel therapeutic strategy utilizing iron chelators. Baillieres Clin Haematol. 1994 Dec;7(4):965–1000. doi: 10.1016/s0950-3536(05)80133-7. [DOI] [PubMed] [Google Scholar]
- Hidalgo E., Demple B. An iron-sulfur center essential for transcriptional activation by the redox-sensing SoxR protein. EMBO J. 1994 Jan 1;13(1):138–146. doi: 10.1002/j.1460-2075.1994.tb06243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H., Emery-Goodman A., Thompson N., Neupert B., Seiser C., Kühn L. C. Expression of active iron regulatory factor from a full-length human cDNA by in vitro transcription/translation. Nucleic Acids Res. 1992 Jan 11;20(1):33–39. doi: 10.1093/nar/20.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirling H., Henderson B. R., Kühn L. C. Mutational analysis of the [4Fe-4S]-cluster converting iron regulatory factor from its RNA-binding form to cytoplasmic aconitase. EMBO J. 1994 Jan 15;13(2):453–461. doi: 10.1002/j.1460-2075.1994.tb06280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. A., Harford J. B. The secondary structure of the regulatory region of the transferrin receptor mRNA deduced by enzymatic cleavage. New Biol. 1992 Apr;4(4):330–338. [PubMed] [Google Scholar]
- Iwai K., Klausner R. D., Rouault T. A. Requirements for iron-regulated degradation of the RNA binding protein, iron regulatory protein 2. EMBO J. 1995 Nov 1;14(21):5350–5357. doi: 10.1002/j.1460-2075.1995.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey S. R., Cohen N. A., Rouault T. A., Klausner R. D., Snyder S. H. The iron-responsive element binding protein: a target for synaptic actions of nitric oxide. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12994–12998. doi: 10.1073/pnas.91.26.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrey S. R., Haile D. J., Klausner R. D., Harford J. B. The interaction between the iron-responsive element binding protein and its cognate RNA is highly dependent upon both RNA sequence and structure. Nucleic Acids Res. 1993 Sep 25;21(19):4627–4631. doi: 10.1093/nar/21.19.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaptain S., Downey W. E., Tang C., Philpott C., Haile D., Orloff D. G., Harford J. B., Rouault T. A., Klausner R. D. A regulated RNA binding protein also possesses aconitase activity. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10109–10113. doi: 10.1073/pnas.88.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoroshilova N., Beinert H., Kiley P. J. Association of a polynuclear iron-sulfur center with a mutant FNR protein enhances DNA binding. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2499–2503. doi: 10.1073/pnas.92.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. Y., Klausner R. D., Rouault T. A. Translational repressor activity is equivalent and is quantitatively predicted by in vitro RNA binding for two iron-responsive element-binding proteins, IRP1 and IRP2. J Biol Chem. 1995 Mar 10;270(10):4983–4986. doi: 10.1074/jbc.270.10.4983. [DOI] [PubMed] [Google Scholar]
- Koeller D. M., Casey J. L., Hentze M. W., Gerhardt E. M., Chan L. N., Klausner R. D., Harford J. B. A cytosolic protein binds to structural elements within the iron regulatory region of the transferrin receptor mRNA. Proc Natl Acad Sci U S A. 1989 May;86(10):3574–3578. doi: 10.1073/pnas.86.10.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeller D. M., Horowitz J. A., Casey J. L., Klausner R. D., Harford J. B. Translation and the stability of mRNAs encoding the transferrin receptor and c-fos. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7778–7782. doi: 10.1073/pnas.88.17.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler S. A., Henderson B. R., Kühn L. C. Succinate dehydrogenase b mRNA of Drosophila melanogaster has a functional iron-responsive element in its 5'-untranslated region. J Biol Chem. 1995 Dec 22;270(51):30781–30786. doi: 10.1074/jbc.270.51.30781. [DOI] [PubMed] [Google Scholar]
- Kühn L. C., McClelland A., Ruddle F. H. Gene transfer, expression, and molecular cloning of the human transferrin receptor gene. Cell. 1984 May;37(1):95–103. doi: 10.1016/0092-8674(84)90304-0. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Laudano A., Yu Y. Structural requirements of iron-responsive elements for binding of the protein involved in both transferrin receptor and ferritin mRNA post-transcriptional regulation. Nucleic Acids Res. 1990 Apr 11;18(7):1819–1824. doi: 10.1093/nar/18.7.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Characterization and evolution of the expressed rat ferritin light subunit gene and its pseudogene family. Conservation of sequences within noncoding regions of ferritin genes. J Biol Chem. 1987 May 25;262(15):7335–7341. [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P. M., Reichert J., Freyd G., Horvitz H. R., Walsh C. T. The LIM region of a presumptive Caenorhabditis elegans transcription factor is an iron-sulfur- and zinc-containing metallodomain. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9210–9213. doi: 10.1073/pnas.88.20.9210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- Lin J. J., Patino M. M., Gaffield L., Walden W. E., Smith A., Thach R. E. Crosslinking of hemin to a specific site on the 90-kDa ferritin repressor protein. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6068–6071. doi: 10.1073/pnas.88.14.6068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins E. A., Robalinho R. L., Meneghini R. Oxidative stress induces activation of a cytosolic protein responsible for control of iron uptake. Arch Biochem Biophys. 1995 Jan 10;316(1):128–134. doi: 10.1006/abbi.1995.1019. [DOI] [PubMed] [Google Scholar]
- May B. K., Bhasker C. R., Bawden M. J., Cox T. C. Molecular regulation of 5-aminolevulinate synthase. Diseases related to heme biosynthesis. Mol Biol Med. 1990 Oct;7(5):405–421. [PubMed] [Google Scholar]
- McClelland A., Kühn L. C., Ruddle F. H. The human transferrin receptor gene: genomic organization, and the complete primary structure of the receptor deduced from a cDNA sequence. Cell. 1984 Dec;39(2 Pt 1):267–274. doi: 10.1016/0092-8674(84)90004-7. [DOI] [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Meyer M., Schreck R., Baeuerle P. A. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 1993 May;12(5):2005–2015. doi: 10.1002/j.1460-2075.1993.tb05850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. J., Earl J. R., Trenam C. W., Blake D. R. Reactive oxygen species and iron--a dangerous partnership in inflammation. Int J Biochem Cell Biol. 1995 Feb;27(2):109–122. doi: 10.1016/1357-2725(94)00084-o. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Kühn L. C. A stem-loop in the 3' untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell. 1988 Jun 3;53(5):815–825. doi: 10.1016/0092-8674(88)90098-0. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Neupert B., Kühn L. C. A specific mRNA binding factor regulates the iron-dependent stability of cytoplasmic transferrin receptor mRNA. Cell. 1989 Jul 28;58(2):373–382. doi: 10.1016/0092-8674(89)90851-9. [DOI] [PubMed] [Google Scholar]
- Müllner E. W., Rothenberger S., Müller A. M., Kühn L. C. In vivo and in vitro modulation of the mRNA-binding activity of iron-regulatory factor. Tissue distribution and effects of cell proliferation, iron levels and redox state. Eur J Biochem. 1992 Sep 15;208(3):597–605. doi: 10.1111/j.1432-1033.1992.tb17224.x. [DOI] [PubMed] [Google Scholar]
- Neupert B., Menotti E., Kühn L. C. A novel method to identify nucleic acid binding sites in proteins by scanning mutagenesis: application to iron regulatory protein. Nucleic Acids Res. 1995 Jul 25;23(14):2579–2583. doi: 10.1093/nar/23.14.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert B., Thompson N. A., Meyer C., Kühn L. C. A high yield affinity purification method for specific RNA-binding proteins: isolation of the iron regulatory factor from human placenta. Nucleic Acids Res. 1990 Jan 11;18(1):51–55. doi: 10.1093/nar/18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira C. C., Goossen B., Zanchin N. I., McCarthy J. E., Hentze M. W., Stripecke R. Translational repression by the human iron-regulatory factor (IRF) in Saccharomyces cerevisiae. Nucleic Acids Res. 1993 Nov 25;21(23):5316–5322. doi: 10.1093/nar/21.23.5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oria R., Sánchez L., Houston T., Hentze M. W., Liew F. Y., Brock J. H. Effect of nitric oxide on expression of transferrin receptor and ferritin and on cellular iron metabolism in K562 human erythroleukemia cells. Blood. 1995 May 15;85(10):2962–2966. [PubMed] [Google Scholar]
- Owen D., Kühn L. C. Noncoding 3' sequences of the transferrin receptor gene are required for mRNA regulation by iron. EMBO J. 1987 May;6(5):1287–1293. doi: 10.1002/j.1460-2075.1987.tb02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K., Gray N. K., Hentze M. W. Differential regulation of two related RNA-binding proteins, iron regulatory protein (IRP) and IRPB. RNA. 1995 Apr;1(2):155–163. [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K., Hentze M. W. Nitric oxide signaling to iron-regulatory protein: direct control of ferritin mRNA translation and transferrin receptor mRNA stability in transfected fibroblasts. Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1267–1271. doi: 10.1073/pnas.92.5.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K., Hentze M. W. Rapid responses to oxidative stress mediated by iron regulatory protein. EMBO J. 1995 Jun 15;14(12):2917–2924. doi: 10.1002/j.1460-2075.1995.tb07291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantopoulos K., Weiss G., Hentze M. W. Nitric oxide and oxidative stress (H2O2) control mammalian iron metabolism by different pathways. Mol Cell Biol. 1996 Jul;16(7):3781–3788. doi: 10.1128/mcb.16.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patino M. M., Walden W. E. Cloning of a functional cDNA for the rabbit ferritin mRNA repressor protein. Demonstration of a tissue-specific pattern of expression. J Biol Chem. 1992 Sep 15;267(26):19011–19016. [PubMed] [Google Scholar]
- Pelicci P. G., Tabilio A., Thomopoulos P., Titeux M., Vainchenker W., Rochant H., Testa U. Hemin regulates the expression of transferrin receptors in human hematopoietic cell lines. FEBS Lett. 1982 Aug 23;145(2):350–354. doi: 10.1016/0014-5793(82)80198-1. [DOI] [PubMed] [Google Scholar]
- Pellat C., Henry Y., Drapier J. C. IFN-gamma-activated macrophages: detection by electron paramagnetic resonance of complexes between L-arginine-derived nitric oxide and non-heme iron proteins. Biochem Biophys Res Commun. 1990 Jan 15;166(1):119–125. doi: 10.1016/0006-291x(90)91919-j. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Haile D., Rouault T. A., Klausner R. D. Modification of a free Fe-S cluster cysteine residue in the active iron-responsive element-binding protein prevents RNA binding. J Biol Chem. 1993 Aug 25;268(24):17655–17658. [PubMed] [Google Scholar]
- Philpott C. C., Klausner R. D., Rouault T. A. The bifunctional iron-responsive element binding protein/cytosolic aconitase: the role of active-site residues in ligand binding and regulation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7321–7325. doi: 10.1073/pnas.91.15.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpott C. C., Rouault T. A., Klausner R. D. Sequence and expression of the murine iron-responsive element binding protein. Nucleic Acids Res. 1991 Nov 25;19(22):6333–6333. doi: 10.1093/nar/19.22.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodromou C., Artymiuk P. J., Guest J. R. The aconitase of Escherichia coli. Nucleotide sequence of the aconitase gene and amino acid sequence similarity with mitochondrial aconitases, the iron-responsive-element-binding protein and isopropylmalate isomerases. Eur J Biochem. 1992 Mar 1;204(2):599–609. doi: 10.1111/j.1432-1033.1992.tb16673.x. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Shapiro D., Mattia E., Bridges K., Klausner R. Effects of alterations in cellular iron on biosynthesis of the transferrin receptor in K562 cells. Mol Cell Biol. 1985 Apr;5(4):595–600. doi: 10.1128/mcb.5.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K., Harford J. B., Rouault T., McClelland A., Ruddle F. H., Klausner R. D. Transcriptional regulation by iron of the gene for the transferrin receptor. Mol Cell Biol. 1986 Jan;6(1):236–240. doi: 10.1128/mcb.6.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson D. R., Neumannova V., Nagy E., Ponka P. The effect of redox-related species of nitrogen monoxide on transferrin and iron uptake and cellular proliferation of erythroleukemia (K562) cells. Blood. 1995 Oct 15;86(8):3211–3219. [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Structure of activated aconitase: formation of the [4Fe-4S] cluster in the crystal. Proc Natl Acad Sci U S A. 1989 May;86(10):3639–3643. doi: 10.1073/pnas.86.10.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. The structure of aconitase. Proteins. 1989;5(4):289–312. doi: 10.1002/prot.340050406. [DOI] [PubMed] [Google Scholar]
- Roberts K. P., Griswold M. D. Characterization of rat transferrin receptor cDNA: the regulation of transferrin receptor mRNA in testes and in Sertoli cells in culture. Mol Endocrinol. 1990 Apr;4(4):531–542. doi: 10.1210/mend-4-4-531. [DOI] [PubMed] [Google Scholar]
- Rothenberger S., Müllner E. W., Kühn L. C. The mRNA-binding protein which controls ferritin and transferrin receptor expression is conserved during evolution. Nucleic Acids Res. 1990 Mar 11;18(5):1175–1179. doi: 10.1093/nar/18.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Haile D. J., Downey W. E., Philpott C. C., Tang C., Samaniego F., Chin J., Paul I., Orloff D., Harford J. B. An iron-sulfur cluster plays a novel regulatory role in the iron-responsive element binding protein. Biometals. 1992 Autumn;5(3):131–140. doi: 10.1007/BF01061319. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Caughman S. W., Harford J. B., Klausner R. D. Binding of a cytosolic protein to the iron-responsive element of human ferritin messenger RNA. Science. 1988 Sep 2;241(4870):1207–1210. doi: 10.1126/science.3413484. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Hentze M. W., Haile D. J., Harford J. B., Klausner R. D. The iron-responsive element binding protein: a method for the affinity purification of a regulatory RNA-binding protein. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5768–5772. doi: 10.1073/pnas.86.15.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tang C. K., Kaptain S., Burgess W. H., Haile D. J., Samaniego F., McBride O. W., Harford J. B., Klausner R. D. Cloning of the cDNA encoding an RNA regulatory protein--the human iron-responsive element-binding protein. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7958–7962. doi: 10.1073/pnas.87.20.7958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego F., Chin J., Iwai K., Rouault T. A., Klausner R. D. Molecular characterization of a second iron-responsive element binding protein, iron regulatory protein 2. Structure, function, and post-translational regulation. J Biol Chem. 1994 Dec 9;269(49):30904–30910. [PubMed] [Google Scholar]
- Schneider C., Owen M. J., Banville D., Williams J. G. Primary structure of human transferrin receptor deduced from the mRNA sequence. Nature. 1984 Oct 18;311(5987):675–678. doi: 10.1038/311675b0. [DOI] [PubMed] [Google Scholar]
- Seiser C., Posch M., Thompson N., Kühn L. C. Effect of transcription inhibitors on the iron-dependent degradation of transferrin receptor mRNA. J Biol Chem. 1995 Dec 8;270(49):29400–29406. doi: 10.1074/jbc.270.49.29400. [DOI] [PubMed] [Google Scholar]
- Seiser C., Teixeira S., Kühn L. C. Interleukin-2-dependent transcriptional and post-transcriptional regulation of transferrin receptor mRNA. J Biol Chem. 1993 Jun 25;268(18):13074–13080. [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Regulation of ferritin mRNA: a possible gene-sparing phenomenon. Induction of ferritin synthesis by iron in liver as well as red cells combines high translational efficiency with increased utilization of preformed ferritin mRNA. J Biol Chem. 1983 Jul 10;258(13):7921–7923. [PubMed] [Google Scholar]
- Shull G. E., Theil E. C. Translational control of ferritin synthesis by iron in embryonic reticulocytes of the bullfrog. J Biol Chem. 1982 Dec 10;257(23):14187–14191. [PubMed] [Google Scholar]
- Shyu A. B., Belasco J. G., Greenberg M. E. Two distinct destabilizing elements in the c-fos message trigger deadenylation as a first step in rapid mRNA decay. Genes Dev. 1991 Feb;5(2):221–231. doi: 10.1101/gad.5.2.221. [DOI] [PubMed] [Google Scholar]
- Sierzputowska-Gracz H., McKenzie R. A., Theil E. C. The importance of a single G in the hairpin loop of the iron responsive element (IRE) in ferritin mRNA for structure: an NMR spectroscopy study. Nucleic Acids Res. 1995 Jan 11;23(1):146–153. doi: 10.1093/nar/23.1.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C., Mitchinson M. J., Aruoma O. I., Halliwell B. Stimulation of lipid peroxidation and hydroxyl-radical generation by the contents of human atherosclerotic lesions. Biochem J. 1992 Sep 15;286(Pt 3):901–905. doi: 10.1042/bj2860901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripecke R., Hentze M. W. Bacteriophage and spliceosomal proteins function as position-dependent cis/trans repressors of mRNA translation in vitro. Nucleic Acids Res. 1992 Nov 11;20(21):5555–5564. doi: 10.1093/nar/20.21.5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stripecke R., Oliveira C. C., McCarthy J. E., Hentze M. W. Proteins binding to 5' untranslated region sites: a general mechanism for translational regulation of mRNAs in human and yeast cells. Mol Cell Biol. 1994 Sep;14(9):5898–5909. doi: 10.1128/mcb.14.9.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson G. R., Walden W. E. Localization of an RNA binding element of the iron responsive element binding protein within a proteolytic fragment containing iron coordination ligands. Nucleic Acids Res. 1994 Jul 11;22(13):2627–2633. doi: 10.1093/nar/22.13.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. K., Chin J., Harford J. B., Klausner R. D., Rouault T. A. Iron regulates the activity of the iron-responsive element binding protein without changing its rate of synthesis or degradation. J Biol Chem. 1992 Dec 5;267(34):24466–24470. [PubMed] [Google Scholar]
- Tardat B., Touati D. Iron and oxygen regulation of Escherichia coli MnSOD expression: competition between the global regulators Fur and ArcA for binding to DNA. Mol Microbiol. 1993 Jul;9(1):53–63. doi: 10.1111/j.1365-2958.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- Testa U., Kühn L., Petrini M., Quaranta M. T., Pelosi E., Peschle C. Differential regulation of iron regulatory element-binding protein(s) in cell extracts of activated lymphocytes versus monocytes-macrophages. J Biol Chem. 1991 Jul 25;266(21):13925–13930. [PubMed] [Google Scholar]
- Toth I., Bridges K. R. Ascorbic acid enhances ferritin mRNA translation by an IRP/aconitase switch. J Biol Chem. 1995 Aug 18;270(33):19540–19544. doi: 10.1074/jbc.270.33.19540. [DOI] [PubMed] [Google Scholar]
- Touati D., Jacques M., Tardat B., Bouchard L., Despied S. Lethal oxidative damage and mutagenesis are generated by iron in delta fur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995 May;177(9):2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Tyrrell R. M. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993 Jul 15;268(20):14678–14681. [PubMed] [Google Scholar]
- Walden W. E., Daniels-McQueen S., Brown P. H., Gaffield L., Russell D. A., Bielser D., Bailey L. C., Thach R. E. Translational repression in eukaryotes: partial purification and characterization of a repressor of ferritin mRNA translation. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9503–9507. doi: 10.1073/pnas.85.24.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden W. E., Patino M. M., Gaffield L. Purification of a specific repressor of ferritin mRNA translation from rabbit liver. J Biol Chem. 1989 Aug 15;264(23):13765–13769. [PubMed] [Google Scholar]
- Ward J. H., Kushner J. P., Kaplan J. Regulation of HeLa cell transferrin receptors. J Biol Chem. 1982 Sep 10;257(17):10317–10323. [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Werner-Felmayer G., Werner E. R., Grünewald K., Wachter H., Hentze M. W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994 Sep 1;180(3):969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K., Munro H. N. Induction of ferritin subunit synthesis by iron is regulated at both the transcriptional and translational levels. J Biol Chem. 1988 Jun 25;263(18):8938–8942. [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Youdim M. B., Ben-Shachar D., Riederer P. The possible role of iron in the etiopathology of Parkinson's disease. Mov Disord. 1993;8(1):1–12. doi: 10.1002/mds.870080102. [DOI] [PubMed] [Google Scholar]
- Yu Y., Radisky E., Leibold E. A. The iron-responsive element binding protein. Purification, cloning, and regulation in rat liver. J Biol Chem. 1992 Sep 15;267(26):19005–19010. [PubMed] [Google Scholar]
- Zheng L., Kennedy M. C., Blondin G. A., Beinert H., Zalkin H. Binding of cytosolic aconitase to the iron responsive element of porcine mitochondrial aconitase mRNA. Arch Biochem Biophys. 1992 Dec;299(2):356–360. doi: 10.1016/0003-9861(92)90287-7. [DOI] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Darl M., Harrison P. M., Bottke W. cDNA cloning and deduced amino acid sequence of two ferritins: soma ferritin and yolk ferritin, from the snail Lymnaea stagnalis L. Eur J Biochem. 1994 Jun 1;222(2):353–366. doi: 10.1111/j.1432-1033.1994.tb18874.x. [DOI] [PubMed] [Google Scholar]