Fig. 1.
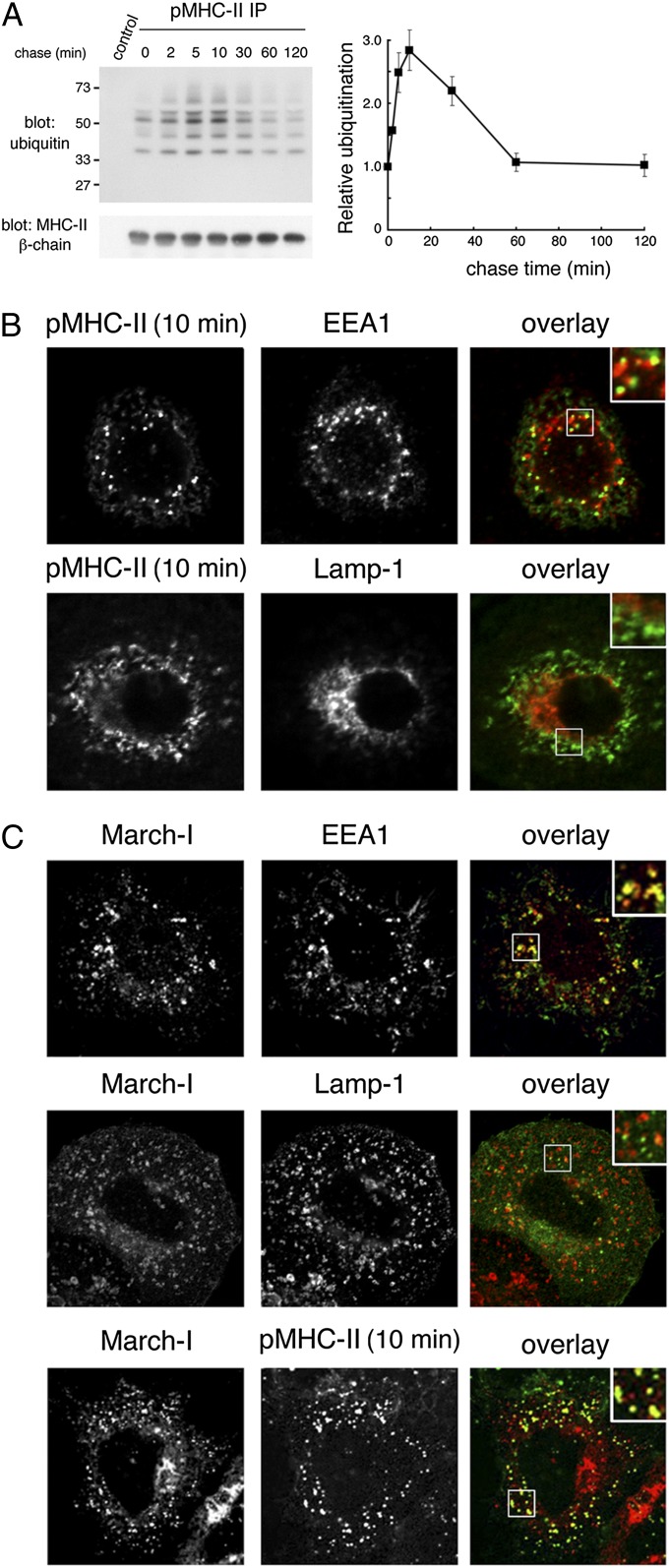
Peptide-loaded MHC-II is ubiquitinated on the plasma membrane and in early endosomes. (A) Human DCs were incubated with anti–pMHC-II mAb L243 or IgG isotype control on ice, washed, and recultured at 37 °C for the indicated times. The cells were lysed in Triton X-100, and mAb L243-bound MHC-II was isolated using protein A–agarose beads. The samples were analyzed by immunoblotting by using anti-ubiquitin or anti–MHC-II β-chain antibodies. The amount of MHC-II ubiquitination and total amount of MHC-II β-chain present at each time point was quantitated by densitometry. MHC-II ubiquitination was normalized to the total amount of MHC-II present in the sample and was expressed relative to the amount of ubiquitination observed at time t 0. The data shown are the mean ± SD obtained from three independent experiments. (B) Human DCs were incubated with anti–pMHC-II mouse mAb L243 for 10 min at 37 °C, and residual cell surface antibodies were blocked by using unlabeled goat anti-mouse IgG serum on ice. The cells were fixed, permeabilized, and stained with Alexa Fluor-conjugated secondary antibody recognizing internalized pMHC-II (L243) and mAb recognizing early endosomes (EEA1) or late endosomes/lysosomes (Lamp-1). (C) HeLa-CIITA cells were transfected with a plasmid encoding V5 epitope-tagged March-I. The cells were incubated with anti–pMHC-II antibody (L243) on ice, washed, and recultured for 10 min at 37 °C. The cells were fixed, permeabilized, and stained with anti-V5 antibody, anti-EEA1 antibody, anti–Lamp-1 antibody, or a secondary antibody allowing detection of internalized pMHC-II mAb L243. (Insets, Right) Enlarged region of the cell.