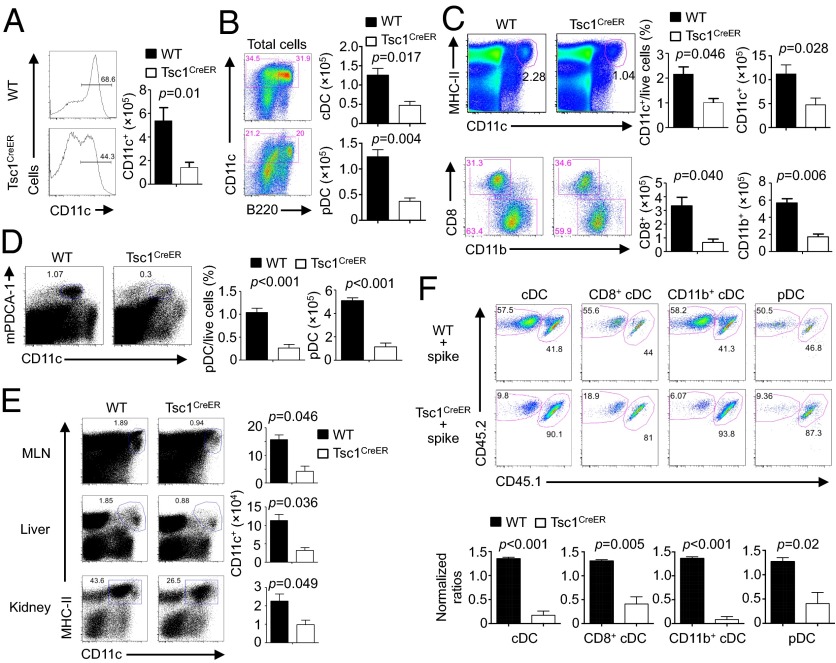
Fig. 1.
Loss of Tsc1 disrupts DC development in vitro and in vivo. (A and B) BM cells from WT or Tsc1CreER mice were cultured with FLT3L (input, 3 × 106) in the presence of 4-hydroxytamoxifen, followed by analysis of the percentage (Left) and cell number of total CD11c+ DCs (Right) (A) and the number of cDCs (CD11c+B220–) and pDCs (CD11c+B220+) (B). (C and D) WT or Tsc1CreER mice were treated with tamoxifen for 5 d, followed by resting for 5–10 d, and the percentage and number of splenic cDCs and cDC subsets (C) or pDCs (D) were analyzed. (E) DC percentage (Left) and number (Right) in mesenteric lymph nodes (MLNs), liver, and kidney of WT or Tsc1CreER mice were analyzed as in C. (F) BM cells from tamoxifen-treated WT or Tsc1CreER CD45.2.2+ mice were mixed with cells from CD45.1.2+ mice at a 1:1 ratio and transferred into irradiated CD45.1.1+ recipients. The contributions of spike BM-derived (CD45.1.2+) and WT or Tsc1CreER donor BM-derived (CD45.2.2+) cells among total CD11c+ cDCs, CD8+ cDCs, CD11b+ cDCs, and pDCs in the reconstituted mixed chimeras were analyzed (Upper). (Lower) Normalized ratios against the percentages of CD4+ T cells. Error bars indicate SEM. Data are representative of two to four independent experiments.