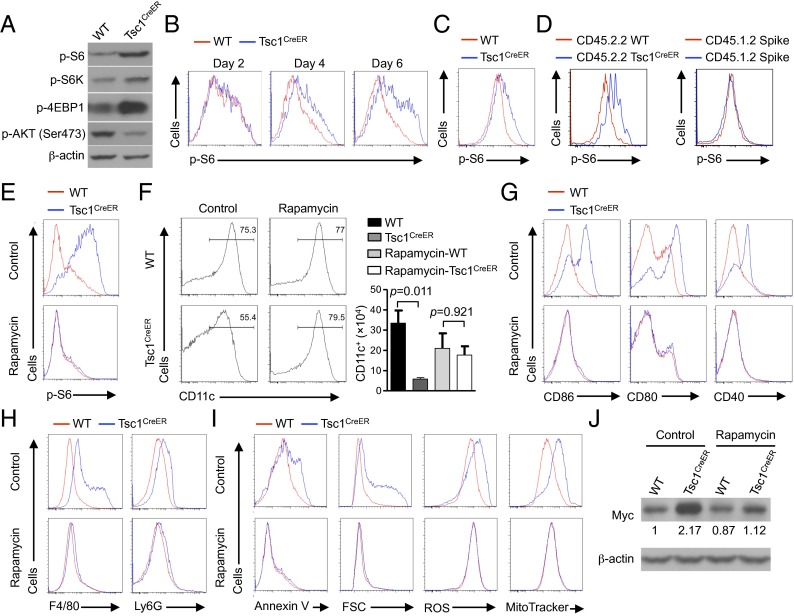
Fig. 6.
Rapamycin-sensitive mTORC1 signaling contributes to Tsc1-deficient DC defects. (A) BM cells from WT or Tsc1CreER mice were cultured with FLT3L and 4-OHT, followed by immunoblot analysis of p-S6, p-S6K, p-4EBP1, and p-AKT (Ser473) in purified CD11c+ cells. (B) BM cells from WT or Tsc1CreER mice were cultured with FLT3L and 4-OHT, followed by flow cytometry analysis of p-S6 at the indicated time points of DC differentiation. (C and D) Phosphorylation of S6 was determined in splenic DCs from tamoxifen-treated WT or Tsc1CreER mice (C) or from WT or Tsc1CreER donor BM-derived cells (Left) and spike BM cells (Right) in the mixed chimeras (generated as described in Fig. 1F) (D). (E–J) BM cells from WT or Tsc1CreER mice were cultured with FLT3L and 4-OHT in the presence of rapamycin or control, followed by analyses of phosphorylation of S6 (E), CD11c+ DC percentage (Left) and number (Right) (input BM cells, 2 × 106) (F), maturation marker expression (G), F4/80 and Ly6G expression (H), Annexin V staining, cell size, ROS production, and mitochondrial mass (I), and Myc expression (the numbers below the lanes indicate the band intensity relative to that of the loading control β-actin) (J). Error bars indicate SEM. Data are representative of two to four independent experiments.