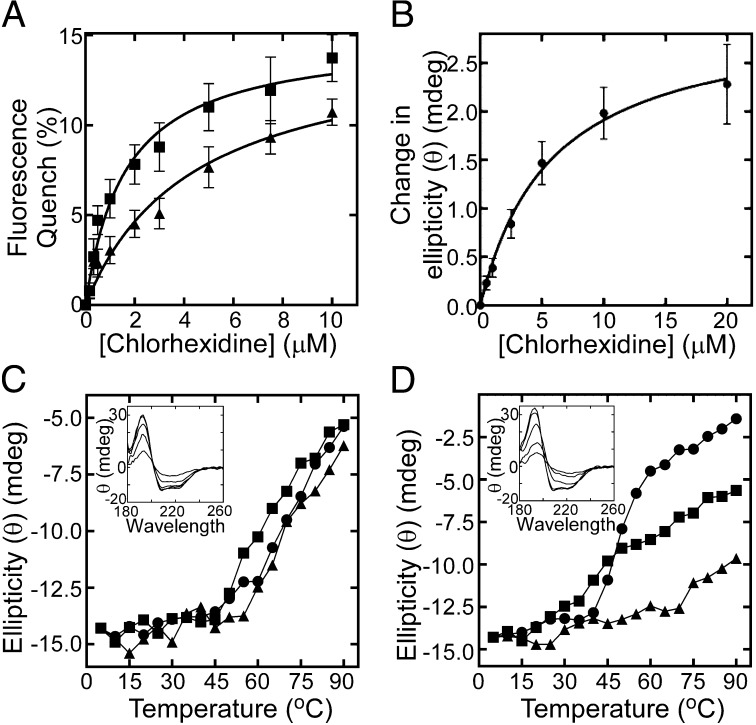
Fig. 4.
AceI–chlorhexidine binding interactions. (A) Trp-fluorescence quenching of 4.4 µM wild-type AceI (■) and E15Q AceI (▲) protein upon chlorhexidine titration. Samples were excited at 295 nm, and the fluorescence emission was measured at 330 nm. The apparent Kd for the wild-type and E15Q AceI-chlorhexidine interactions determined via nonlinear regression were 1.6 µM and 4.4 µM, respectively. (B) Average change in ellipticity (θ) (mdeg) of 20 µM wild-type AceI protein across wavelengths 258–280 nm during titration of chlorhexidine. The apparent Kd determined for the AceI–chlorhexidine interaction in this experiment was 5.8 µM. Error bars show the SEM ellipticity change from different wavelengths. (C and D) Thermal stability of 33 µM wild-type (C) and E15Q (D) AceI protein in the presence and absence of chlorhexidine as determined by synchrotron radiation CD spectrophotometry. The ellipticity at 209 nm is shown for protein only (●), protein plus 100 µM chlorhexidine (■; 1:3 molar ratio) and protein plus 500 µM chlorhexidine (▲; 1:15 molar ratio) at increasing temperature. Insets show the characteristic α-helical protein far-UV spectrum of the respective proteins at increasing temperature in the absence of chlorhexidine.