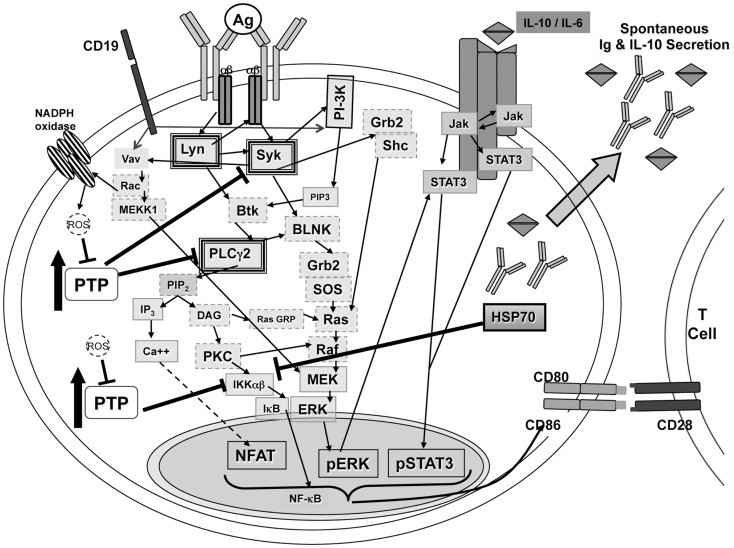
Figure 4.
Signaling in B-1a cells. B-1a cells constitutively secrete IgM and IL-10 without prior stimulation. In addition, B-1a cells have constitutive levels of activated ERK, STAT3, and NF-AT, yet are not able to activate NF-κB in response to BCR ligation. It has been shown constitutive ERK activation in B-1 cells is the result of chronic signaling through the BCR (51). The constitutive ERK activation was shown to be dependent upon src kinases, PI-3K, Syk, and PLCγ2, which are heavily outlined in the illustration. Inhibition of PI-3K or Syk also blocked the constitutive levels of CD86 on B-1a cells, which is known to play an essential role during allogeneic stimulation of T cells (23, 51). All mediators outlined in a dashed gray line may play a role in signal transduction leading to constitutive ERK activation but have not been tested. Phosphatase activity, denoted as PTP, has been shown to control phosphorylation of Syk and PLCγ2 differentially in B-1a cells as compared to B2 cells (51). In addition, inhibition of phosphatase activity in B-1 cells was shown, Figure 2, to allow IκBα degradation in B-1a cells after BCR ligation. Therefore, PTP activity in B-1a cells inhibits NF-κB activation by an unknown mechanism. It is hypothesized that this mechanism involves HSP70 and IL-10, which were both shown to be expressed at a higher level in naive B-1a cells as compared to naive B2 cells, Figure 3 and Ref. (24). Furthermore, it has been shown that inhibition of constitutively active Lyn allowed for partial recovery of B-1a cells’ responsiveness to BCR ligation (38). In addition, suboptimal Vav levels in B-1a cells (35) may not be sufficient for the production of ROS, which are necessary to inhibit phosphatases to allow activation of NF-κB.