Abstract
The alignment and mobility of the cervical spine is influenced by factors related to the vertebral bodies, intervertebral discs, ligaments, facet joints, and muscles. Few reports have described the role played by the paraspinal muscles in cervical spine mobility. In this study, we investigate the relationship between fatty degeneration of the paraspinal muscles and cervical motion as assessed with kinetic magnetic resonance imaging (kMRI). One hundred eighty-eight symptomatic patients underwent cervical kMRI in neutral, flexion, and extension positions. We quantified cervical paraspinal muscle fatty infiltration and measured angular variation and translational motion at each cervical level, and the global Cobb angle. Cervical paraspinal muscle fatty degeneration demonstrated a pattern in which C3 and C7 had significantly more fatty infiltration than C4, C5, and C6. Additionally, when the normal group was compared with the fatty degeneration group with respect to angular variation, translational motion, and Cobb angle, no significant differences were found except in angular variation at the C3–C4 level. In conclusion, we found a significantly larger quantity of fatty degeneration in the paraspinal muscles at C3 and C7 than the middle cervical levels. Also, we demonstrate that fatty degeneration does not significantly affect cervical lordotic alignment or mobility characteristics.
Keywords: kinematic magnetic resonance imaging, cervical spine, cervical paraspinal muscle, cervical lordosis, fatty degeneration, fatty infiltration, multifidus muscle
Vertebral bodies, intervertebral discs, spinal ligaments, facet joints, and muscles make up the components of the cervical spine, which moves through flexion, neutral, and extension positions. As with other spine and skeletal components, muscles play an important role in cervical spine stability, and there is evidence that muscle atrophy is associated with neck pain.1 2
To this point, no study has examined the contribution of paraspinal muscles to the kinematics or motion characteristics of the cervical spine. Many authors have investigated the role of the paraspinal muscles in lumbar spine motion. Techniques that have been used include histological analysis,3 4 5 measurement of muscular strength with force-measuring devices,3 measurement of the electromyographic signal,6 7 measurement of muscle cross-sectional area by ultrasound scanning, measurement of muscle cross-sectional area using computed tomography (CT) scans8 9 10 and magnetic resonance imaging (MRI),3 11 and measurement of muscular density by CT8 9 10 and MRI.11 12 13 In particular, MRI provides noninvasive and reproducible information on soft tissue structures such as intervertebral discs, spinal ligaments, extensor muscles, and neural elements. For the evaluation of muscle, MRI has been widely used and is the diagnostic modality of choice. In investigating cervical paraspinal musculature, MRI has allowed the quantification of extensor muscle atrophy and fatty infiltration in a patient population of whiplash-associated disorders.14
The purpose of this study was to investigate the relationship between cervical paraspinal muscle fatty degeneration and the kinematics of the cervical spine functional motion unit. It was hypothesized that fatty degeneration in the cervical multifidus muscles would affect cervical alignment and mobility characteristics.
Materials and Methods
Patient Population
From April 2011 to September 2011, patients symptomatic for neck pain or radiculopathy underwent cervical kinetic MRI (kMRI) in neutral, flexion, and extension positions. Subjects included 95 men and 93 women with a mean age of 53.2 years (range 21 to 61 years). None of the subjects had previously undergone spine surgery or received a diagnosis of deformity. The Institutional Review Board at our institution approved this study.
kMRI Technique
kMRIs of the cervical spine were acquired from a 0.6 Tesla MRI scanner (Upright Multi-Position; Fonar Corp., New York, NY) in upright weight-bearing neutral, flexion, and extension positions, using a flexible surface coil. The magnets are separated by a 0.5-mm gap. A standard imaging protocol was used, which included sagittal T1-weighted spin-echo sequences [repetition time (TR)/echo time (TE), 671/17 milliseconds; slice thickness, 3.0 mm; field of view, 24 cm; matrix, 256 × 200; and number of excitations (NEX), 2] and T2-weighted fast spin-echo sequences (TR/TE, 3432/160 milliseconds; slice thickness, 3.0 mm; field of view, 24 cm; and NEX, 2). Axial T2-weighted spin-echo sequences were only acquired with fat suppression.
kMRI Analysis
All MRI parameters were digitized using computer guidance software, operated by experienced spine surgeons. All calculations were performed by MRAnalyzer Version 2.0 (Truemetric Corp., Bellflower, CA) kMRI analysis software as described in previous publications.15 16 Midsagittal images were selected from flexion, neutral, and extension MRI series. Each image was marked for digitization from the occiput to T1 by spine surgeons as guided by computer software (occiput: 2 points on the anterior and posterior baselines; C1: 4 points on the anterior, superior, and inferior surfaces of the anterior tubercle and the inferior pole of the spinous process; C2: 1 point on the tip of the odontoid process and 2 on the pedicle; C3–1: 6 points on each vertebral body, 2 on each pedicle, and 2 on the spinal canal diameter at each intervertebral disc level; total: 77 points).
MRAnalyzer computer software measures directly from the digitized images and calculates associated kinematic and alignment parameters geometrically. The basic measurements for evaluation of stability include intervertebral angular displacement and translation as assessed between flexion, neutral, and extension positions. Finally, total mobility (translational motion and angular variation) was calculated as the difference between flexion and extension for each parameter at each cervical level. Translational motion was measured at five cervical intervertebral disc levels from C2/C3 to C6/C7 by determining the anteroposterior motion of the superior and inferior vertebrae relative to each other. By convention, in our data, a positive value was used to indicate anterior translation of the superior vertebra over the inferior vertebra (anterolisthesis); a negative value was used to indicate posterior translation (retrolisthesis). Intervertebral angular variation was also measured at the same five levels. The calculation of angular variation involved the extrapolation of lines drawn connecting the inferior corners of adjacent vertebral bodies and extending them to a point of intersection. By convention, if the intersection occurred posteriorly, the angle formed was designated a negative sign and indicated lordotic curvature. Similarly, anterior intersections were designated positive angles and indicated kyphosis. In addition, the C1–C7 Cobb angle was measured in flexion, neutral, and extension positions. This angle is made by drawing a line through the atlas plane from the center point of the anterior tubercle through the posterior arch. A second line is constructed through the lower epiphyseal plate of C7, with both lines converging posteriorly. Perpendiculars to these two lines were constructed and the angle was measured.
Assessment of Paraspinal Muscle Fatty Degeneration
All MRIs were analyzed with ImageJ (version 1.45, http://rsbweb.nih.gov/ij/) image processing computer software. The measurement protocol included manually tracing regions of interest around each of the bilateral cervical multifidus muscles as anatomically identified on both axial T1- and T2-weighted images at each of five vertebral levels (C3 to C7). The axial MRI selected for C3 was the slice available through the most inferior aspect of the vertebral body. The slice selected for C4 to C7 was the one through the most superior aspect of the vertebral body (C4 to C7), as described previously in assessment of cervical multifidus muscle fatty degeneration by axial magnetic resonance series.14 Our team measured multifidus muscle area once without fat inclusion using T1-weighted images and a second time with fat inclusion in the muscle area using fat suppression T2-weighted images (Fig. 1). Fat area was calculated as the difference, and quantification of fatty infiltration was made by dividing fat area by whole muscle area.
Figure 1.
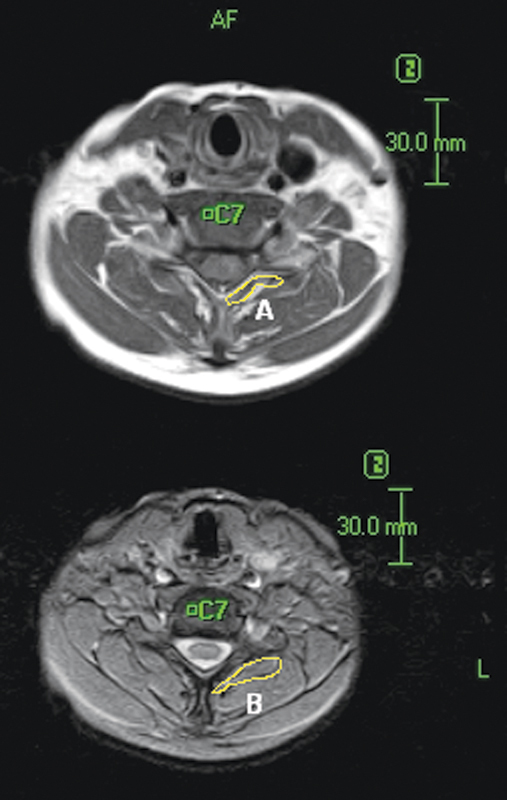
Manual tracing of region of interest bounded by the multifidus muscles at the level of the C7 inferior vertebral body on T1 (lower frame) and T2 (upper frame) weighted magnetic resonance images. Fatty infiltration = (B–A)/B.
Cervical levels were classified into two groups according to the degree of fatty degeneration: group A: normal, fatty infiltration less than 25%; group B: fatty degeneration, fatty infiltration greater than 25%. A modified classification system was used based upon the previously reported system of classifying fatty degeneration in lumbar paraspinal muscle according to degenerative changes in the lumbar multifidus muscle.17 18
One experienced observer measured all multifidus muscle areas. Intraobserver reliability was assessed by repeating the measurements of 10 randomly selected patients (50 multifidus areas). The significance of any differences between the original and the repeat data was assessed by calculating the intraclass correlation coefficient (ICC).
Assessment of Disc Degeneration Grade
Cervical disc degeneration grade was assigned based upon a comprehensive system reported previously.15 16 Three independent spine surgeons classified the 1128 cervical intervertebral discs from 188 patients into five grades (Table 1) based upon the neutral-position T2-weighted sagittal images. Interobserver repeatability was calculated using ICC to be 0.85, suggesting good repeatability.
Table 1. Grading System for Cervical Intervertebral Disc Degeneration.
| Grade | Nucleus Signal Intensity | Nucleus Structure | Distinction of Nucleus and Annulus | Disc Height |
|---|---|---|---|---|
| I | Hyperintense | Homogenous, white | Clear | Normal |
| II | Hyperintense | Inhomogeneous with horizontal band, white | Clear | Normal |
| III | Intermediate | Inhomogeneous, gray to black | Unclear | Normal to decreased |
| IV | Hypointense | Inhomogeneous, gray to black | Lost | Normal to decreased |
| V | Hypointense | Inhomogeneous, gray to black | Lost | Collapsed |
Statistical Analysis
Statistical analyses were performed using SPSS (version 17; SPSS, Chicago, IL) computer software. Mann-Whitney U test was used for statistical analyses. ICC values for intra- and interobserver repeatability were calculated. A p value of less than 0.05 was considered statistically significant.
Results
Fatty Degeneration of Cervical Paraspinal Muscle
Fatty degeneration was measured as described above and reported as the mean fat index at each cervical level. The repeatability assessment showed good agreement between the original and repeat data for the multifidus muscle area measurements (ICC 0.85), indicating that the original measures were reliable. The mean fat index of the multifidus muscle for each level is shown in Fig. 2. The fat content of the multifidus muscle was significantly higher (p < 0.001) at the upper and lower levels of the cervical spine, C3 and C7, with average fat indices of 0.32 and 0.31, as compared with middle cervical levels C4, C5, and C6, which had fat indices of 0.27, 0.25, and 0.26, respectively.
Figure 2.
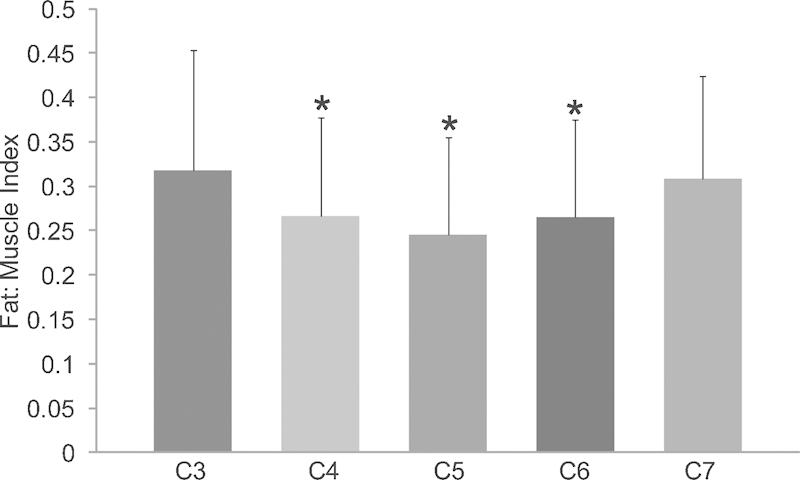
Mean differences of the fat indices in the cervical multifidus muscles across segmental vertebral levels (C3 to C7). The fat indices of the multifidus muscle at C3 and C7 were larger than C4, C5, and C6 (p < 0.001). *Indicates a significant difference when compared with C3 and C7.
Cervical Kinematics
Angular variation was then measured at the upper and lower levels of each segment (Fig. 3). At C3, lower-level angular variation was significantly higher in group A (4.24 ± 3.11) when compared with group B (3.58 ± 3.85; p < 0.05). However, there were no significant differences in angular variation between any other levels. With respect to translational motion, no significant difference was observed between group A and group B. For example, the average translational motion at the upper level of C3 was 1.3 mm in group A and 1.29 mm in group B. A similar pattern was seen at all levels with regard to translational motion.
Figure 3.
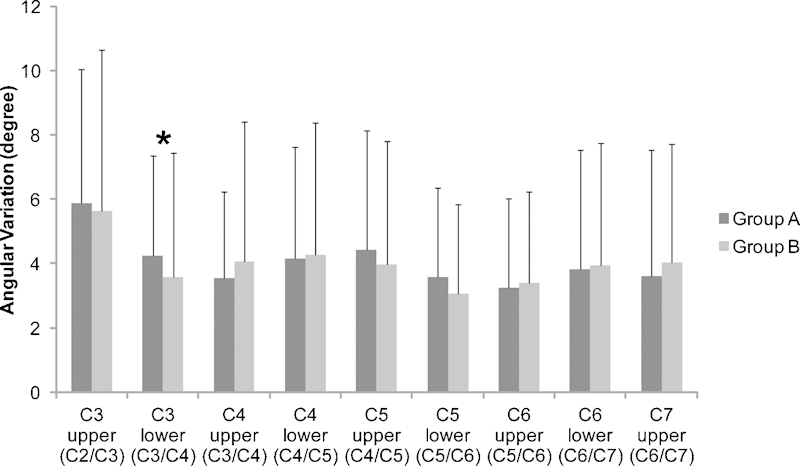
Angular variation of each cervical unit. The fatty infiltration of the multifidus muscles at each cervical level was compared with the angular variation of the upper and lower level. There was a significant difference between group A and group B with regard to the variation at the C3 lower level. (A) Normal; (B) fatty degeneration. *Indicates a significant difference between groups A and B (p < 0.05).
In addition to measuring translational and angular variation between group A and group B at each cervical level, the global Cobb angle from C1 to C7 was also measured in each patient and correlated with the fatty degeneration at each level. No correlation was found between fatty infiltration at a given level (classified as group A or B) and a change in the global Cobb angle of the patient.
Fatty Degeneration and Disc Degeneration
The distribution of grades of disc degeneration for all patients is listed for each group in Fig. 4. At the C4 upper level, the grade of disc degeneration was significantly higher in group A (3.66 ± 0.60) when compared with group B (3.43 ± 0.58), although this difference was small. At almost every level, no significant difference was observed in the grade of disc degeneration between group A and group B.
Figure 4.
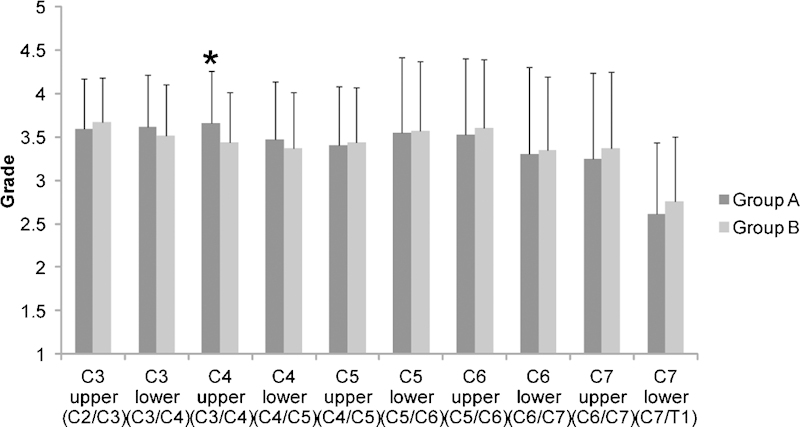
Disc degeneration of each cervical unit. The fatty infiltration of the multifidus muscle at each cervical level was compared with the level of disc degeneration. There was a significant difference between group A and group B with regard to the variation at the C4 upper level. (A) Normal; (B) fatty degeneration. *Indicates a significant difference between groups A and B (p < 0.05).
Discussion
This study showed that using MRI to measure fatty infiltration19 is an effective way to assess changes within the cervical paraspinal extensors, particularly the multifidus. Short deep extensor muscles (such as the multifidus) have been shown to function in the maintenance of cervical spine stability in various biomechanical models.20 Patients with whiplash-associated disorders were evaluated in a similar study that showed multifidus muscle fat content to be significantly higher at each spinal level tested compared with the other cervical paraspinal muscles including the semispinalis cervicis, semispinalis capitis, splenius capitis, and upper trapezius.14 The multifidus muscle has a direct attachment to the cervical facet capsule, and as such, it may contribute more significantly to head mobility, neck posture, cervical injury, and neck pain syndromes.2
In our study, the cervical multifidus muscle demonstrated significantly more fatty infiltration at C3 and C7 than at C4, C5, and C6. Previously, Elliot et al14 had studied healthy controls and reported a primarily linear decrease in fatty infiltrate content of the multifidus muscle progressing from C3 to C7 down the cervical spine. In contrast to healthy controls, there was a significantly higher fatty infiltrate content at C3 than the more caudal multifidi measured at C4, C5, C6, and C7 (p < 0.0001) in the experimental group containing patients with whiplash-associated disorders. In our current study of patients undergoing cervical kMRI for symptomatic neck pain or radiculopathy, we found that fatty infiltration occurred both in the upper and lower cervical spine, a pattern slightly different than that observed in Elliot's study of symptomatic whiplash patients.
We found that angular variation demonstrated no significant differences between the normal and fatty degeneration groups with the exception of the comparison at the lower level of C3, which had a significant difference (p < 0.05). In addition, translational motion and Cobb angle also demonstrated no significant differences between the normal group and the fatty degeneration group. Thus, our data demonstrate that fatty degeneration of the cervical multifidus muscle causes little change in segmental motion and cervical lordosis.
With regard to disc degeneration in the cervical spine, there were also no significant differences between the normal group and the fatty degeneration group, with the exception of the upper level of C4 (p < 0.05). This difference, though significant, was small, representing a difference in disc degeneration grading of less than 0.2. This result suggests that fatty degeneration in the cervical multifidus muscle has little effect on disc degeneration.
Using a porcine model of spine disc degeneration in vitro, Cheng et al21 investigated the relationship between cervical muscle recruitment and cervical spine stability in animals with both intact and degenerative cervical spines to test the hypothesis that muscle that is dysfunctional from fatty change is not as efficient in stabilizing the spine. They found that spinal stability was more affected by muscle dysfunction than by disc degeneration. This is contradictory to our findings that Cobb angle, translational motion, and angular variation (except at the C3 lower level) demonstrate no significant differences between the normal group and the fatty degeneration group. Our findings that spinal stability is affected more by disc degeneration than by muscle dysfunction appears reasonable given that a major portion of axial load is sustained by the intervertebral discs.22
In conclusion, we demonstrated that fatty degeneration of the C3 and C7 paraspinal muscles is significantly greater than middle segment (C4–C6) paraspinal muscle fatty degeneration. We hypothesized that fatty degeneration of cervical paraspinal muscle is associated with changes in cervical lordosis and cervical movement. However, fatty degeneration had almost no affect on cervical lordosis or segmental movement. This information is important clinically as there is very little information in the literature on the contribution, if any, of the paraspinal musculature to cervical spine motion and stability. This further adds to our understanding of the contribution of the muscles, ligaments, intervertebral discs, and bony structures of the cervical spine to its motion.
Acknowledgments
The authors thank Megan Cory and Timothy Chai for their technical support.
Disclosures
Hirokazu Inoue, None
Scott Montgomery, None
Bayan Aghdasi, None
Yanlin Tan, None
Haijun Tian, None
Xiong Jian, None
Rodney Terrell, None
Vijay Singh, None
Jeffrey C. Wang, Royalties: Medtronics, Stryker, Seaspine, Osprey, Aesculap, Biomet, Amedica, Zimmer, Synthes; Stock Ownership: Fziomed; Private Investments: Promethean Spine, Paradigm Spine, Benevenue, NexGen, K2 Medical, Pioneer, Amedica, Vertiflex, Electrocore, Surgitech, Axiomed; Board of Directors: North American Spine Society, Cervical Spine Research Society, AO Spine/AO Foundation; Scientific Advisory Board: VG Innovations, Corespine, Expanding Orthopaedics, Syndicom, Osprey, Amedica, Bone Biologics, Curative Biosciences, PearlDiver, Inc., Pioneer, Seaspine
References
- 1.McPartland J M, Brodeur R R, Hallgren R C. Chronic neck pain, standing balance, and suboccipital muscle atrophy—a pilot study. J Manipulative Physiol Ther. 1997;20:24–29. [PubMed] [Google Scholar]
- 2.Anderson J S, Hsu A W, Vasavada A N. Morphology, architecture, and biomechanics of human cervical multifidus. Spine. 2005;30:E86–E91. doi: 10.1097/01.brs.0000153700.97830.02. [DOI] [PubMed] [Google Scholar]
- 3.Käser L, Mannion A F, Rhyner A, Weber E, Dvorak J, Müntener M. Active therapy for chronic low back pain: part 2. Effects on paraspinal muscle cross-sectional area, fiber type size, and distribution. Spine. 2001;26:909–919. doi: 10.1097/00007632-200104150-00014. [DOI] [PubMed] [Google Scholar]
- 4.Taylor H, McGregor A H, Medhi-Zadeh S. et al. The impact of self-retaining retractors on the paraspinal muscles during posterior spinal surgery. Spine. 2002;27:2758–2762. doi: 10.1097/00007632-200212150-00004. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchi Y, Matsui H, Tsuji H. Back muscle injury after posterior lumbar spine surgery. A histologic and enzymatic analysis. Spine. 1996;21:941–944. doi: 10.1097/00007632-199604150-00007. [DOI] [PubMed] [Google Scholar]
- 6.Tong H C, Haig A J, Yamakawa K S, Miner J A. Paraspinal electromyography: age-correlated normative values in asymptomatic subjects. Spine. 2005;30:E499–E502. doi: 10.1097/01.brs.0000176318.95523.12. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey A R, Nargol A V, Jones A P, Ratcliffe A A, Greenough C G. The value of electromyography of the lumbar paraspinal muscles in discriminating between chronic-low-back-pain sufferers and normal subjects. Eur Spine J. 2005;14:175–184. doi: 10.1007/s00586-004-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keller A, Gunderson R, Reikerås O, Brox J I. Reliability of computed tomography measurements of paraspinal muscle cross-sectional area and density in patients with chronic low back pain. Spine. 2003;28:1455–1460. doi: 10.1097/01.BRS.0000067094.55003.AD. [DOI] [PubMed] [Google Scholar]
- 9.Storheim K, Holm I, Gunderson R, Brox J I, Bø K. The effect of comprehensive group training on cross-sectional area, density, and strength of paraspinal muscles in patients sick-listed for subacute low back pain. J Spinal Disord Tech. 2003;16:271–279. doi: 10.1097/00024720-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Danneels L A, Vanderstraeten G G, Cambier D C, Witvrouw E E, De Cuyper H J. CT imaging of trunk muscles in chronic low back pain patients and healthy control subjects. Eur Spine J. 2000;9:266–272. doi: 10.1007/s005860000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyun J K, Lee J Y, Lee S J, Jeon J Y. Asymmetric atrophy of multifidus muscle in patients with unilateral lumbosacral radiculopathy. Spine. 2007;32:E598–E602. doi: 10.1097/BRS.0b013e318155837b. [DOI] [PubMed] [Google Scholar]
- 12.Hu Z J, He J, Zhao F D, Fang X Q, Zhou L N, Fan S W. An assessment of the intra- and inter-reliability of the lumbar paraspinal muscle parameters using CT scan and magnetic resonance imaging. Spine. 2011;36:E868–E874. doi: 10.1097/BRS.0b013e3181ef6b51. [DOI] [PubMed] [Google Scholar]
- 13.Lee J C, Cha J G, Kim Y, Kim Y I, Shin B J. Quantitative analysis of back muscle degeneration in the patients with the degenerative lumbar flat back using a digital image analysis: comparison with the normal controls. Spine. 2008;33:318–325. doi: 10.1097/BRS.0b013e318162458f. [DOI] [PubMed] [Google Scholar]
- 14.Elliott J, Jull G, Noteboom J T, Darnell R, Galloway G, Gibbon W W. Fatty infiltration in the cervical extensor muscles in persistent whiplash-associated disorders: a magnetic resonance imaging analysis. Spine. 2006;31:E847–E855. doi: 10.1097/01.brs.0000240841.07050.34. [DOI] [PubMed] [Google Scholar]
- 15.Miyazaki M, Hong S W, Yoon S H. et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine. 2008;33:187–193. doi: 10.1097/BRS.0b013e3181604501. [DOI] [PubMed] [Google Scholar]
- 16.Morishita Y, Hida S, Miyazaki M. et al. The effects of the degenerative changes in the functional spinal unit on the kinematics of the cervical spine. Spine. 2008;33:E178–E182. doi: 10.1097/BRS.0b013e318166f059. [DOI] [PubMed] [Google Scholar]
- 17.Le Huec J C, Basso Y, Aunoble S, Friesem T, Bruno M B. Influence of facet and posterior muscle degeneration on clinical results of lumbar total disc replacement: two-year follow-up. J Spinal Disord Tech. 2005;18:219–223. [PubMed] [Google Scholar]
- 18.Kong M H, Hymanson H J, Song K Y. et al. Kinetic magnetic resonance imaging analysis of abnormal segmental motion of the functional spine unit. J Neurosurg Spine. 2009;10:357–365. doi: 10.3171/2008.12.SPINE08321. [DOI] [PubMed] [Google Scholar]
- 19.Elliott J M, Galloway G J, Jull G A, Noteboom J T, Centeno C J, Gibbon W W. Magnetic resonance imaging analysis of the upper cervical spine extensor musculature in an asymptomatic cohort: an index of fat within muscle. Clin Radiol. 2005;60:355–363. doi: 10.1016/j.crad.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Cholewicki J, McGill S M. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon) 1996;11:1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 21.Cheng C H, Chen P J, Kuo Y W, Wang J L. The effects of disc degeneration and muscle dysfunction on cervical spine stability from a biomechanical study. Proc Inst Mech Eng H. 2011;225:149–157. doi: 10.1243/09544119JEIM805. [DOI] [PubMed] [Google Scholar]
- 22.Broberg K B. On the mechanical behaviour of intervertebral discs. Spine. 1983;8:151–165. doi: 10.1097/00007632-198303000-00006. [DOI] [PubMed] [Google Scholar]


