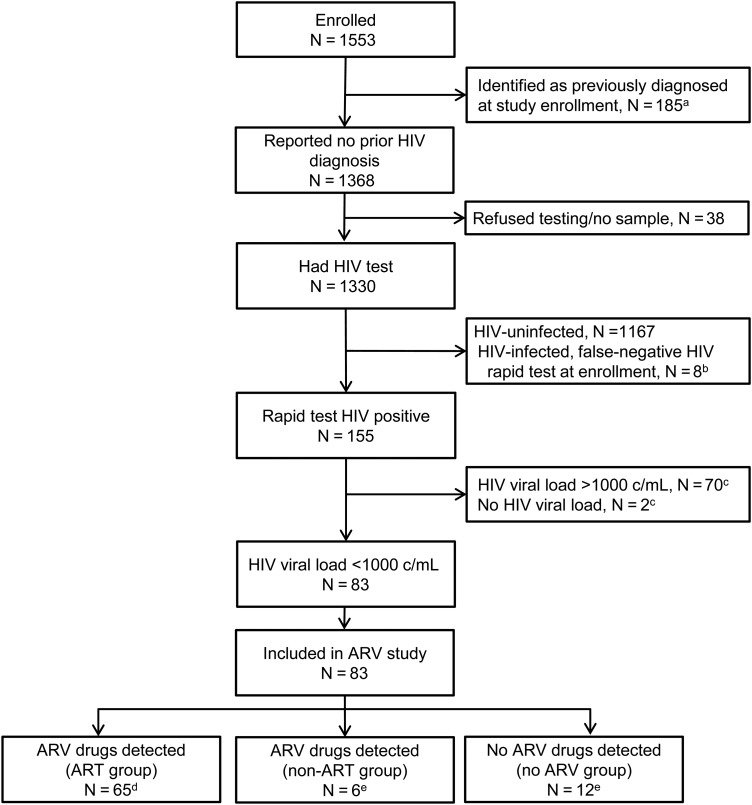
Figure 1.
Selection of study participants for antiretroviral (ARV) drug screening in HPTN 061. aIncludes 150 men who reported a prior diagnosis of human immunodeficiency virus (HIV) and 24 men who were identified as HIV infected in the HPTN 061 study (from review of public records or HIV testing). Eleven of these men reported ARV drug use. bHIV infection was identified retrospectively for these 8 men (based on retrospective testing performed at the HPTN Network Laboratory); they were not included in the ARV study. cMen were not included in the ARV study if they had a viral load >1000 copies/mL at enrollment or if viral load data were not available from the enrollment visit. dThese men had a pattern of ARV drugs detected that was consistent with ARV treatment. The classification for these men was changed from newly diagnosed (provisional) to previously diagnosed based on ARV drug test results. eThe classification for these men was not changed; their final classification was newly diagnosed. Abbreviations: ART, antiretroviral therapy; ARV, antiretroviral; c/mL, copies per milliliter; HIV, human immunodeficiency virus.