Figure 2. Strategies to improve adoptive T-cell immunotherapy.
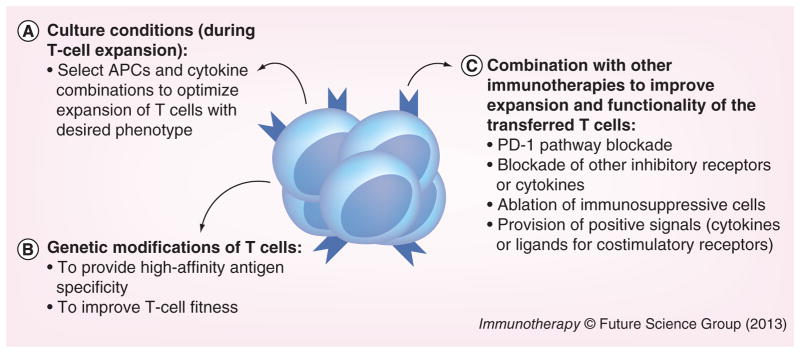
We propose that adoptive T-cell immunotherapy could be improved with: (A) optimization of culture conditions for ex vivo T-cell expansion in order to generate T cells with the desired phenotype. Novel types of APCs, as well as cytokine combinations have been able to generate functional T cells with specific phenotypes. (B) Genetic modifications of T cells, to generate T cells with high affinity to the desired antigen (by introducing a specific T-cell receptor or even chimeric antigen receptor) or with special features (e.g., for HIV-infected patients, T cells that are deficient in HIV coreceptors can be generated). (C) Combination with other immunotherapies to improve in vivo expansion and function of the transferred T cells. Chronically infected hosts or cancer patients have several immunosuppressive mechanisms in place that dampen immune responses. Thus, to overcome inhibitory signals and avoid exhaustion of the transferred T cells, adoptive immunotherapy should be combined with other strategies that remove immunosuppressive pathways (e.g., blockade of the PD-1 pathway). Nevertheless, to be able to improve adoptive immunotherapy at the steps we propose, future studies will need to define the ideal T-cell specificity and phenotype to be transferred (which will vary according to the infection/cancer to be treated). In addition, an analysis of the immunosuppressive mechanisms in place in each patient may guide which pathways need to be modulated to increase the efficiency of adoptively transferred T cells as well as improve endogenous responses.
