Table 7.
Alkynylation with Reduced Stoichiometry.
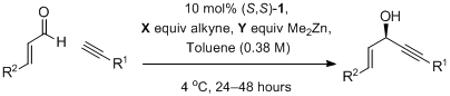
| Alkyne R1 | R2 | X/Y | comments[a] | yield[b] | ee[c] | |
|---|---|---|---|---|---|---|
|
| ||||||
| 1 | CO2Me | iPr | 3.0/3.0 | - | 97% | 97% |
| 2 | CO2Me | iPr | 1.5/1.5 | - | 79% | 97% |
| 3 | CO2Me | Ph | 1.2/1.5 | 0.48 M | 81%d | 94% |
| 4 | CO2Me | Ph | 1.2/0.2 | 60 °C, 24 h | - | - |
| 5 | (CH2)5CH3 | Ph | 3.0/3.0 | - | 83% | 81% |
| 6 | (CH2)5CH3 | Ph | 1.5/1.5 | - | 60% | 75% |
| 7 | (CH2)5CH3 | Ph | 1.2/1.5 | 20 mol % TPPO, 0.48 M |
80% | 93% |
| 8 | TMS | iPr | 3.0/3.0 | - | 100% | 94% |
| 9 | TMS | iPr | 1.2/1.2 | - | 71% | 50% |
| 10 | TMS | Ph | 3.0/3.0 | - | 75% | 90% |
| 11 | TMS | Ph | 1.2/1.2 | - | 52%[e] | 50% |
| 12 | TMS | Ph | 1.2/1.5 | 20 mol % TPPO, 0.48 M |
83% | 88% |
Reaction concentration is reported with respect to alkyne.
Isolated yield.
Enantiomeric excess determined by chiral HPLC analysis.
Isolated along with 16% yield of the methyl addition side product.
17% recovered starting material.
