Table 8.
Optimization of Alkyne Addition with an Enolizable Substrate.
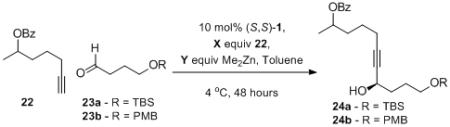
| CHO | X/Y | conditions | yield[a] | ee[b] | |
|---|---|---|---|---|---|
|
| |||||
| 1 | 23a | 2.8/2.95 | - | 35%[c] | 45% |
| 2 | 23a | 2.8/2.95 | 20 mol % TPPO | 39% | 62% |
| 3 | 23a | 1.2/1.5 | 20 mol % TPPO | 22% | 54% |
| 4 | 23a | 1.2/1.3 | 30 mol % NMI | 11% | 72% |
| 5 | 23a | 1.2/1.4 | 4 equiv DMSO | 4% | 14% |
| 6 | 23a | 1.2/1.4 | 4 equiv DMF | 10% | 17% |
| 7 | 23b | 2.8/2.95 | 20 mol % TPPO, 24 hour alkyne premix |
58% | 50%[d] |
| 8 | 23b | 2.8/2.95 | 20 mol % (S,S)-1, 40 mol % TPPO, 24 hour alkyne premix |
69% | 67%[d] |
Isolated yield. All reactions were run on a 0.1625 mmol scale.
Enantiomeric excess determined by chiral HPLC analysis.
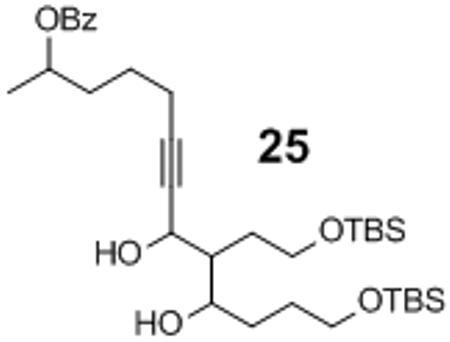
Isolated along with 19% yield of the cross aldol side product 25
Enantiomeric excess determined by 1H-NMR analysis of the corresponding (S)-methyl mandelate.