Abstract
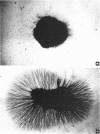
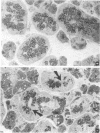
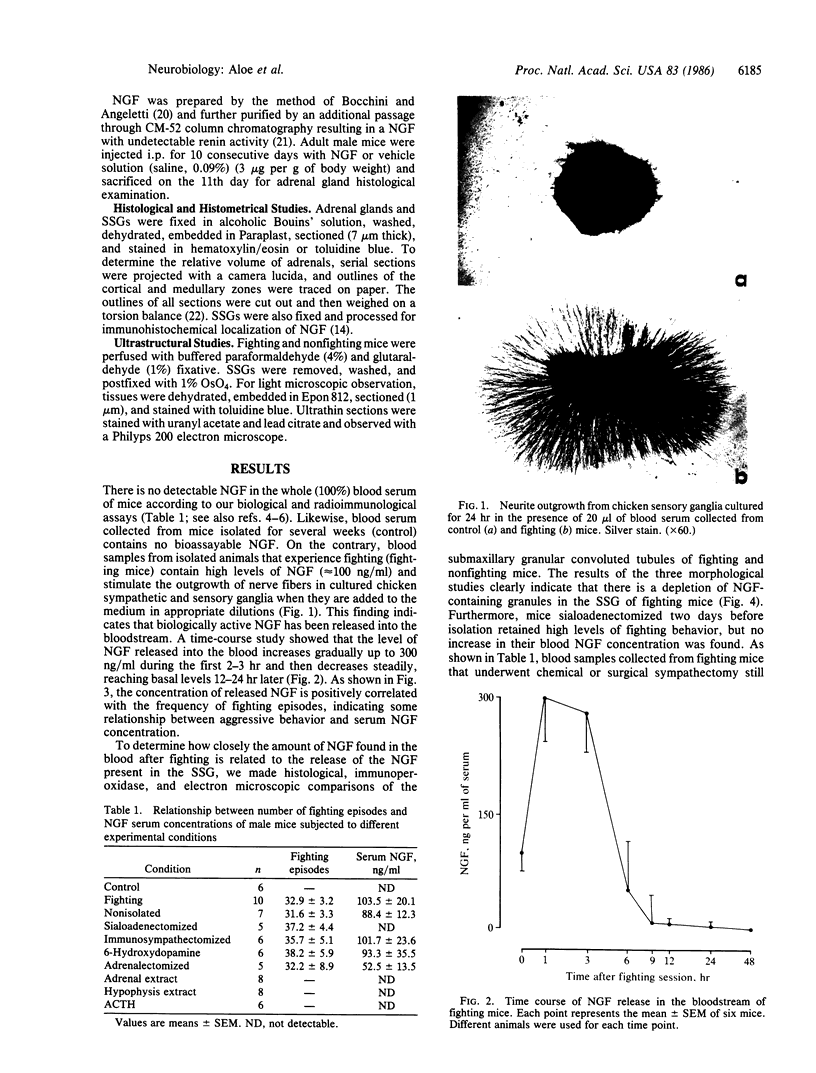
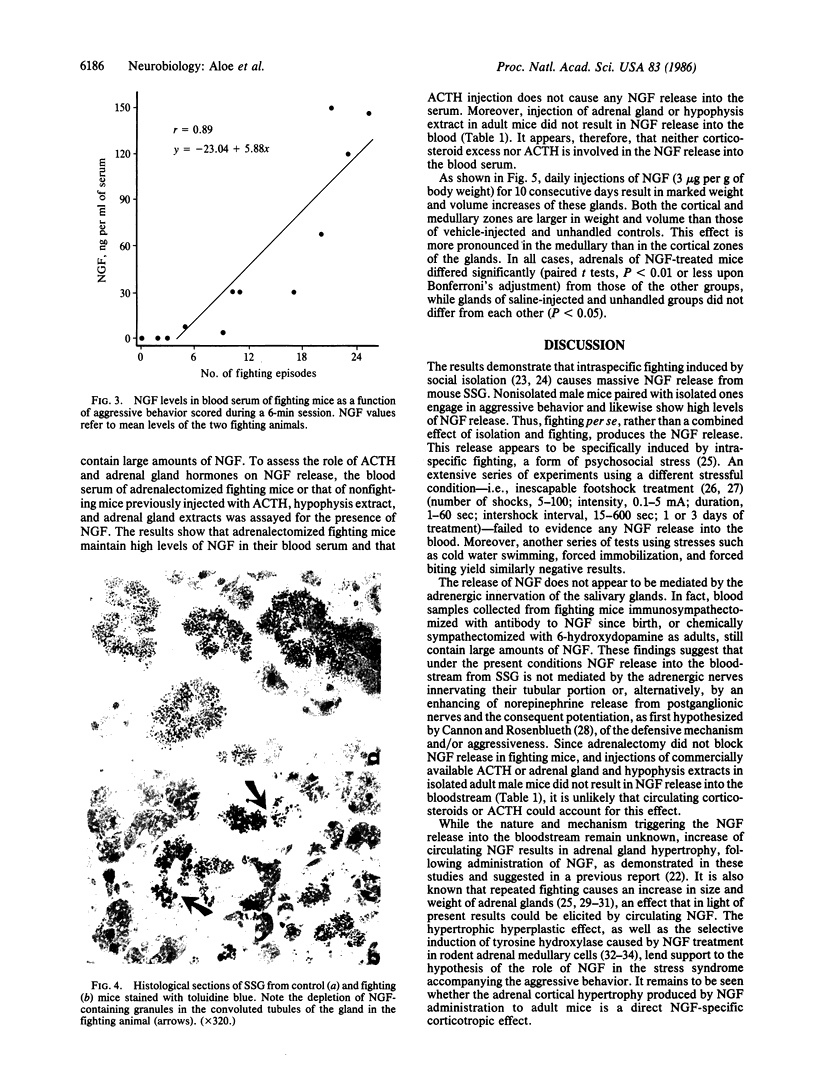
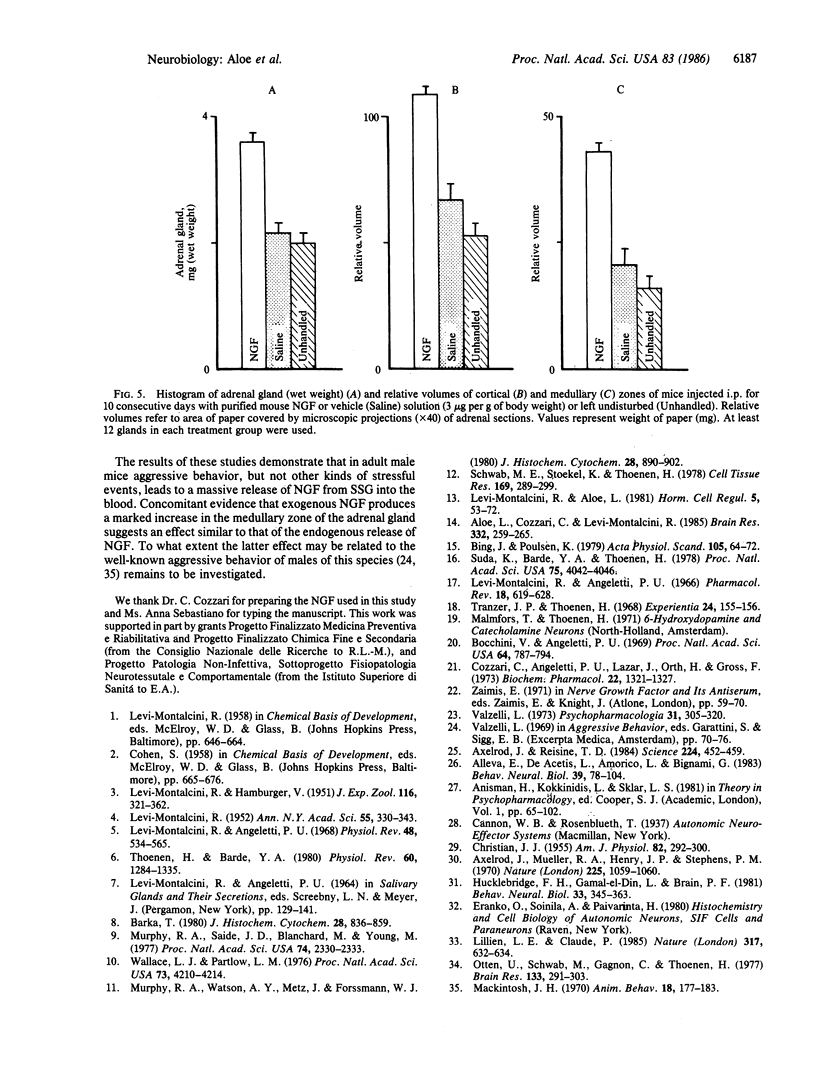
Intraspecific fighting induced by 6-8 weeks of social isolation results in massive release of nerve growth factor (NGF) into the bloodstream of adult male mice. The amount of circulating NGF is highly correlated with the number of fighting episodes. Biological, radioimmunological, immunohistochemical, and ultrastructural studies show that NGF is discharged from the salivary gland into the blood within minutes after fighting and reaches the highest level 3-4 hr later. Adrenergic innervation of the salivary gland or adrenalectomy does not abolish the NGF release. Corticotropic hormones do not induce NGF increase in the blood. Daily administrations of highly purified NGF (3 micrograms per g of body weight) result in a considerable increase in the volume of adrenal glands. These findings are unequivocable evidence for a physiological role of the mouse salivary glands as a major source of blood NGF.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alleva E., de Acetis L., Amorico L., Bignami G. Amphetamine, conditioned stimulus, and nondebilitating preshock effects on activity and avoidance: further evidence for interactions between associative and nonassociative changes. Behav Neural Biol. 1983 Sep;39(1):78–104. doi: 10.1016/s0163-1047(83)90654-4. [DOI] [PubMed] [Google Scholar]
- Aloe L., Cozzari C., Levi-Montalcini R. Cyclocytidine-induced release of nerve growth factor from mouse submandibular glands enhances regeneration of sympathetic fibers in adult mice. Brain Res. 1985 Apr 22;332(2):259–265. doi: 10.1016/0006-8993(85)90595-5. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Mueller R. A., Henry J. P., Stephens P. M. Changes in enzymes involved in the biosynthesis and metabolism of noradrenaline and adrenaline after psychosocial stimulation. Nature. 1970 Mar 14;225(5237):1059–1060. doi: 10.1038/2251059a0. [DOI] [PubMed] [Google Scholar]
- Axelrod J., Reisine T. D. Stress hormones: their interaction and regulation. Science. 1984 May 4;224(4648):452–459. doi: 10.1126/science.6143403. [DOI] [PubMed] [Google Scholar]
- Barka T. Biologically active polypeptides in submandibular glands. J Histochem Cytochem. 1980 Aug;28(8):836–859. doi: 10.1177/28.8.7003006. [DOI] [PubMed] [Google Scholar]
- Bing J., Poulsen K. In mice aggressive behavior provokes vast increase in plasma renin concentration, causing only slight, if any, increase in blood pressure. Acta Physiol Scand. 1979 Jan;105(1):64–72. doi: 10.1111/j.1748-1716.1979.tb06315.x. [DOI] [PubMed] [Google Scholar]
- Bocchini V., Angeletti P. U. The nerve growth factor: purification as a 30,000-molecular-weight protein. Proc Natl Acad Sci U S A. 1969 Oct;64(2):787–794. doi: 10.1073/pnas.64.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIAN J. J. Effect of population size on the adrenal glands and reproductive organs of male mice in populations of fixed size. Am J Physiol. 1955 Aug;182(2):292–300. doi: 10.1152/ajplegacy.1955.182.2.292. [DOI] [PubMed] [Google Scholar]
- Cozzari C., Angeletti P. U., Lazar J., Orth H., Gross F. Separation of isorenin activity from nerve growth factor (NGF) activity in mouse submaxillary gland extracts. Biochem Pharmacol. 1973 Jun 1;22(11):1321–1327. doi: 10.1016/0006-2952(73)90306-7. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R. Effects of mouse tumor transplantation on the nervous system. Ann N Y Acad Sci. 1952 Aug 8;55(2):330–344. doi: 10.1111/j.1749-6632.1952.tb26548.x. [DOI] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., HAMBURGER V. Selective growth stimulating effects of mouse sarcoma on the sensory and sympathetic nervous system of the chick embryo. J Exp Zool. 1951 Mar;116(2):321–361. doi: 10.1002/jez.1401160206. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Second symposium on catecholamines. Modification of sympathetic function. Immunosympathectomy. Pharmacol Rev. 1966 Mar;18(1):619–628. [PubMed] [Google Scholar]
- Lillien L. E., Claude P. Nerve growth factor is a mitogen for cultured chromaffin cells. Nature. 1985 Oct 17;317(6038):632–634. doi: 10.1038/317632a0. [DOI] [PubMed] [Google Scholar]
- Murphy R. A., Saide J. D., Blanchard M. H., Young M. Nerve growth factor in mouse serum and saliva: role of the submandibular gland. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2330–2333. doi: 10.1073/pnas.74.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. A., Watson A. Y., Metz J., Forssmann W. G. The mouse submandibular gland: an exocrine organ for growth factors. J Histochem Cytochem. 1980 Aug;28(8):890–902. doi: 10.1177/28.8.6969274. [DOI] [PubMed] [Google Scholar]
- Otten U., Schwab M., Gagnon C., Thoenen H. Selective induction of tyrosine hydroxylase and dopamine beta-hydroxylase by nerve growth factor: comparison between adrenal medulla and sympathetic ganglia of adult and newborn rats. Brain Res. 1977 Sep 16;133(2):291–303. doi: 10.1016/0006-8993(77)90765-x. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Stöckel K., Thoenen H. Immunocytochemical localization of nerve growth factor (NGF) in the submandibular gland of adult mice by light and electron microscopy. Cell Tissue Res. 1976 Jun 28;169(3):289–299. doi: 10.1007/BF00219602. [DOI] [PubMed] [Google Scholar]
- Suda K., Barde Y. A., Thoenen H. Nerve growth factor in mouse and rat serum: correlation between bioassay and radioimmunoassay determinations. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4042–4046. doi: 10.1073/pnas.75.8.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Barde Y. A. Physiology of nerve growth factor. Physiol Rev. 1980 Oct;60(4):1284–1335. doi: 10.1152/physrev.1980.60.4.1284. [DOI] [PubMed] [Google Scholar]
- Tranzer J. P., Thoenen H. An electron microscopic study of selective, acute degeneration of sympathetic nerve terminals after administration of 6-hydroxydopamine. Experientia. 1968 Feb 15;24(2):155–156. doi: 10.1007/BF02146956. [DOI] [PubMed] [Google Scholar]
- Valzelli L. The "isolation syndrome" in mice. Psychopharmacologia. 1973 Aug 3;31(4):305–320. doi: 10.1007/BF00421275. [DOI] [PubMed] [Google Scholar]
- Wallace L. J., Partlow L. M. alpha-Adrenergic regulation of secretion of mouse saliva rich in nerve growth factor. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4210–4214. doi: 10.1073/pnas.73.11.4210. [DOI] [PMC free article] [PubMed] [Google Scholar]