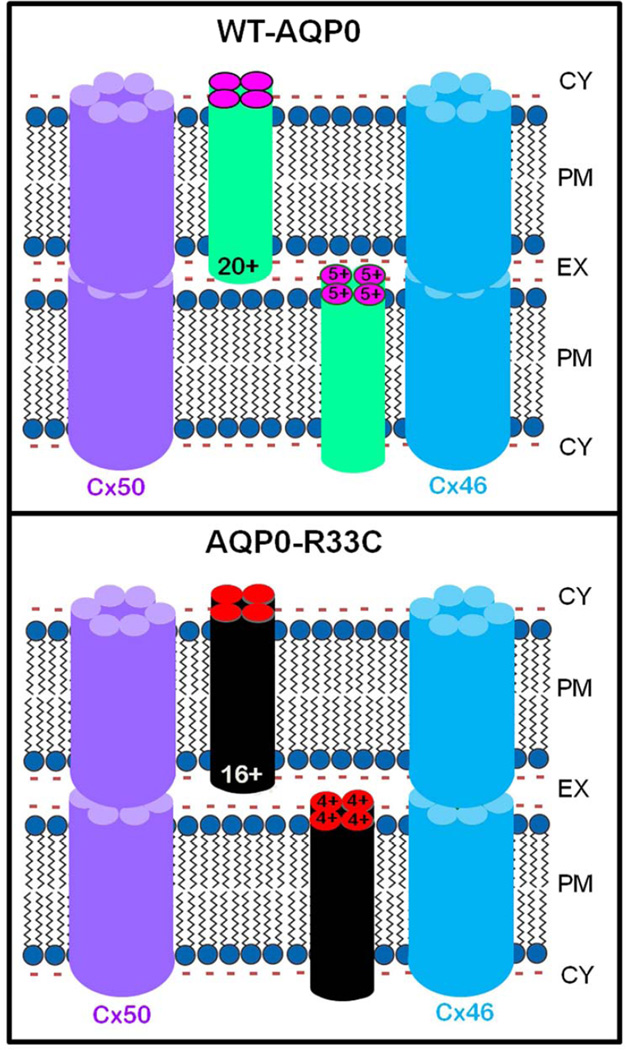
Fig. 8.
Schematic models illustrating the possible cause for autosomal dominant congenital lens cataract due to R33C missense mutation. WT-AQP0: It has been postulated that positive charges in the extracellular loops of AQP0 play a significant role in cell-to-cell adhesion. WT-AQP0 monomers form tetramers. Each tetramer carries a total of 20 positive charges (5 / monomer) in Extracellular Loops, A, C and E. These positive charges electrostatically attract negatively charged lipids of the opposing fiber cell plasma membrane to come closer, thus reducing the extracellular space between the fibers and enabling firm and tight cell-to-cell adhesion. The proximity of fiber cells facilitates gap junction hemichannels to couple and form channels for intercellular communication, which aids in microcirculation and homeostasis of the avascular lens. R33C-AQP0: Mutation at codon 33 in the Extracellular Loop A of AQP0 resulted in replacement of positively charged amino acid arginine (R) to a neutral amino acid cysteine (C). Mutant AQP0-R33C tetramer has only 16 positive charges in extracellular loops due to loss of an arginine to cysteine in each monomer. This reduction might have decreased the pulling and holding forces between opposing fiber cells due to the fewer number of positive charges interacting with negative charges of the plasma membrane lipids, leading to wider intercellular space between adjacent fibers compared to that in the WT-AQP0. Reduced cell-to-cell adhesion of AQP0-R33C might have eventually caused loss of gap junction coupling, compromising intercellular communication, microcirculation and homeostasis of the lens.