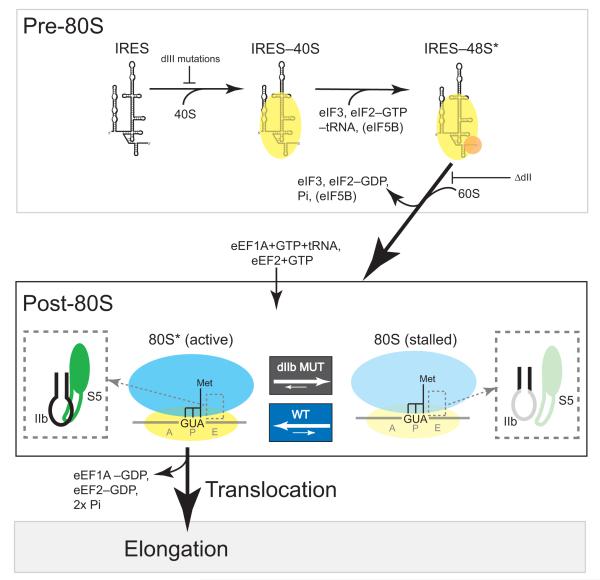
Figure 7. Model of domain IIb (dIIb)’s role in HCV IRES translation initiation.
Top box: simplified pathway for HCV IRES-driven translation initiation up to the point of 80S ribosome formation. This process includes 40S subunit binding, ternary complex (eIF2-met-tRNAi-GTP) binding, eIF3 binding, and then subunit joining and factor release. Below this are the post-80S events that we hypothesize occur within the newly formed 80S ribosome. Briefly, dIIb interacts with the β hairpin of rpS5 (dashed box: dIIb in black, rpS5 in green) and this favors a conformation that is fully competent to accept a tRNA into the A site (delivery catalyzed by eukaryotic elongation factor 1A, eIF1A) and subsequent translocation (catalyzed by eEF2). We term this fully competent conformation “80S*”. When dIIb is mutated (right), the local structure of the dIIb apical loop shifts towards an inactive conformation and the productive interaction with rpS5 is lost (dashed box: dIIb in gray, rpS5 in light green). This favors an 80S ribosome state that stalls prior to translocation. In our model, the active (80S*) and inactive (80S) states are in dynamic equilibrium and the presence of an intact dIIb shifts the equilibrium towards 80S*, thus promoting progression to elongation.