Fig. 2.
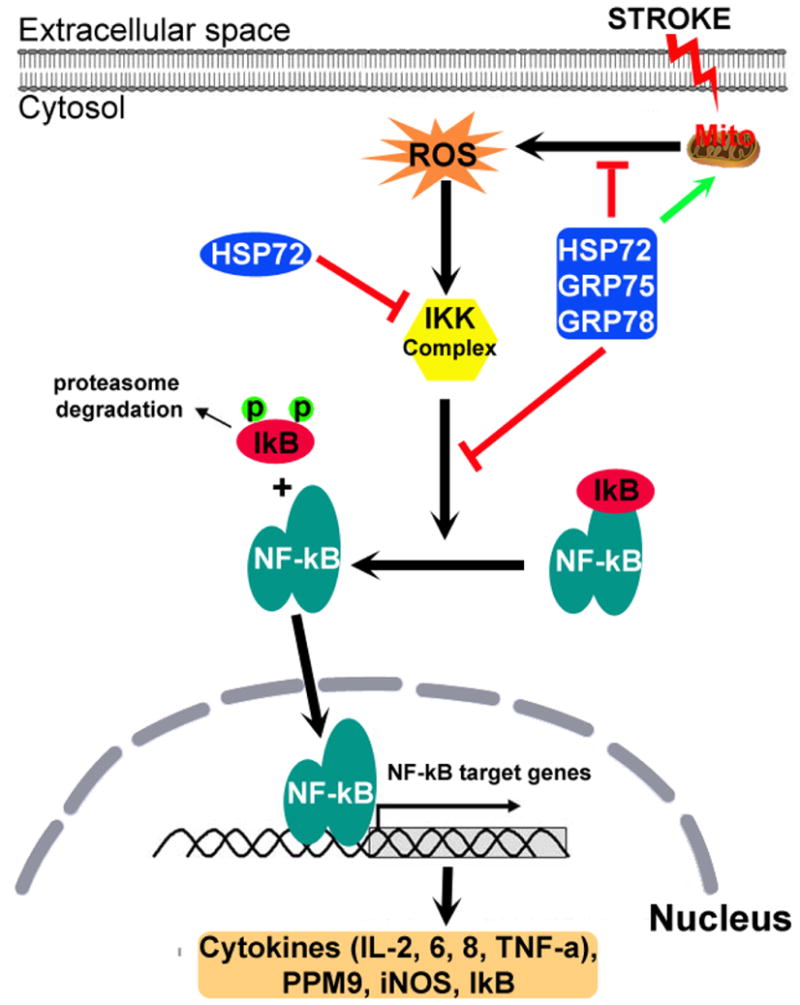
Chaperone machinery influences the NF-kB pro-inflammatory signaling pathway. NF-kB, a dimer consisting of p50/p65 subunits is normally resident in the cytosol and is maintained in an inactive form by its inhibitor IkB. Stroke stimulates mitochondria to release reactive oxygen species (ROS) that activate the IkB kinase (IKK) complex. The activated IKK complex phosphorylates IkB and initiates its ubiquitination and degradation, exposing the nuclear localization signal on NF-kB. NF-kB then translocates to the nucleus and binds to the promoter region of genes expressing pro-inflammatory cytokines and IkB. HSP72 interacts with the IKK complex and several HSP70 family members (HSP70, GRP75 and GRP78), protects mitochondrial function, inhibits ROS production and NF-kB activation.
