Pulmonary arterial hypertension (PAH) is a disorder characterized by elevated vascular resistance in pulmonary arterioles. Progressive increases in pulmonary vascular resistance and pulmonary artery pressures result in right heart failure and reduced cardiac output. Patients experience progressive exertional dyspnea, right heart failure, syncope, and ultimately death. The common pathophysiological features of PAH include pulmonary vasoconstriction, intimal and smooth muscle proliferation, in situ thrombosis, and pathological remodeling of pulmonary arterial circulation. While the origin of PAH is multifactorial, impairments in vasodilator (nitric oxide and prostaglandin signaling) and vasoconstrictor (endothelin-1, reactive oxygen species, angiotensin II) pathways underlie the evolution of early disease.1 Based on this knowledge, drugs that enhance the NO signaling pathways (phosphodiesterase 5 inhibitors), the prostenoids, and endothelin receptor blockers, have been developed and approved for PAH specific therapy.
Inhaled nitric oxide (NO) gas can alleviate vasoconstriction and may modulate cellular proliferative responses, but NO therapy is limited by the need for continuous inhalation, NO reactions with oxygen to form nitrogen dioxide, and special delivery devices. It is now appreciated that inorganic nitrite and nitrate are bio-transformed to NO via the nitrate-to-nitrite-to-NO pathway,2 leading to studies with inhaled nitrite as an alternative to NO gas inhalation.3, 4 In this issue of Circulation, Baliga and colleagues investigate the nitrate-to-nitrite-to-NO pathway by studying the effects of oral nitrite and nitrate on pre-clinical mouse and rat models of PAH, and then attempt to characterize the enzymes that regulate bioconversion of nitrite to NO. They find that both nitrate and nitrite delivered in drinking water can prevent and reverse experimental PAH in the hypoxic and bleomycin mouse models; consistent with published models for in vivo conversion of nitrite to NO.5-7 They also provide unexpected evidence that eNOS may have nitrite reductase activity in the diseased lung, with experiments demonstrating that eNOS null mice with hypoxic PH do not significantly respond to oral nitrate and nitrite treatment.
The therapeutic effects of nitrate and nitrite have been investigated in several disease models (recently reviewed2). Nitrite can induce vasodilation, reduce blood pressure, act as a cytoprotectant in ischemia-reperfusion induced injury, and modulate mitochondrial respiration, energetics and exercise efficiency.2, 8, 9 Nitrate also mediates these effects via conversion to nitrite in the oral cavity by commensal bacteria containing efficient nitrate reductase enzyme systems.10-12
In PAH sheep, mouse, and rat models nitrite acts as a pulmonary vasodilator and produces therapeutic vascular remodeling. Inhalation of nebulized nitrite decreases pulmonary arterial pressure induced by hypoxia and thromboxane4 and can prevent or reverse established right ventricle (RV) pressure increases, RV hypertrophy, and pulmonary vascular remodeling in hypoxia- or monocrotaline- induced PAH.3 Similarly, injection of sodium nitrite attenuates PAH induced by monocrotaline,13 hypoxia, and/or thromboxane.2, 14 Baliga and colleagues now report the effect of oral nitrite and nitrate on hypoxia-induced PAH. Their data demonstrate that oral nitrate or nitrite treatment decrease RV pressure, RV mass, and pulmonary vascular remodeling induced by hypoxia and bleomycin. The protective effects of oral nitrate and nitrite administration correlate to elevated plasma nitrite and cGMP concentrations, providing a potential orally-active therapy for PAH via the nitrate-nitrite-NO pathway.
Mammals can utilize two pathways to produce NO and regulate blood pressure and blood flow: the well-established arginine-to-NO pathway and the more recently characterized nitrate-to-nitrite-to-NO pathway.2 In the arginine-to-NO pathway, the nitric oxide synthase (NOS) enzymes catalyze NO production. The nitrate-to-nitrite-to-NO pathway is less completely understood, as the molecular mechanisms involved in each interconversion and in each organ system are yet to be fully elucidated. However, the upstream pathways for conversion of nitrate to nitrite have been clearly defined since early studies first described this pathways involvement in stomach NO level regulation, shown to be important in regulating stomach mucosal integrity, mucosal host defense, and later mucosal blood flow.10, 12, 15 The two NO biosynthesis pathways respond to oxygen concentration differently: the arginine-to-NO pathway requires oxygen as a substrate for NO formation; conversely, the nitrate-to-nitrite-to-NO pathway is oxygen independent and is actually potentiated under hypoxic conditions (Figure 1). Because the nitrate-to-nitrite-to-NO pathway exhibits increased potency under lower oxygen tensions, a role for nitrite as an effector of hypoxic signaling and vasodilation has been considered.2, 5, 7
Figure 1.
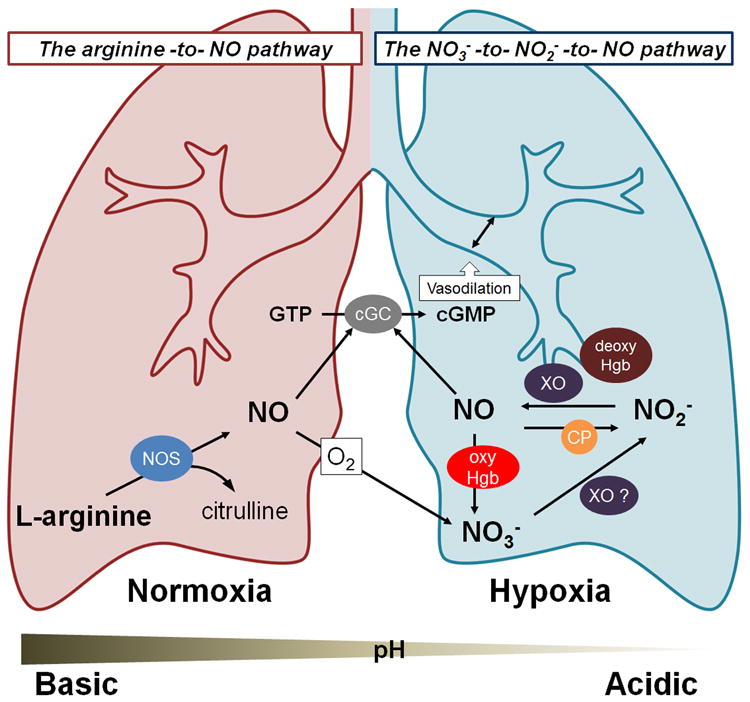
Nitrite (NO2−), and nitrate (NO3−) can treat PAH via biotransformation into nitric oxide (NO), a potent vasodilator. The nitrate-nitrite-NO pathway dominates under hypoxia (blue lung), and the arginine-NO pathway under normoxia (red lung). Abbreviations: nitric oxide synthase (NOS); xanthine oxidoreductase (XO); deoxyhemoglobin (deoxyHgb); oxyhemoglobin (oxyHgb); ceruloplasmin (CP); guanosine triphosphate (GTP); cytosolic guanylate cyclase (cGC); cyclic guanosine monophosphate (cGMP).
Eating nitrate rich foods, such as spinach or beets, increases nitrate levels in saliva, as high as 8 mM. Approximately one quarter of this nitrate is transformed by bacterial nitrate reductases to nitrite, which is subsequently swallowed and then transformed into NO, which is important for host defense, and mucosal integrity and blood flow.10, 12, 15 The enterosalivary conversion of nitrate to nitrite requires oral bacteria and is inhibited in germ free mice and with antibiotic or antiseptic therapy.16 Liver xanthine oxidoreductase (XO) has been reported to convert nitrate to nitrite under certain conditions; however, compared to the bacterial transformation, the XO catalyzed reaction is inefficient, and a clear pathway for nitrate reduction in mammalian organs remains uncertain. In the last decade it has become clear that nitrite not only modulates stomach mucosal function, but is an important intravascular source of NO. Arterial-to-venous nitrite gradients within the human circulation lead to early hypotheses that nitrite could be a source of NO in the human circulation.5 Later studies confirmed that nitrite exhibited potent vasodilatory effects in vivo.7 Since these initial studies, over 10 years ago, the role of nitrite in regulation of blood flow and pressure at physiological levels has been clearly demonstration by many research groups.17, 18 The signaling properties of nitrite are largely mediated by nitrite reduction to NO, which then activates soluble guanylate cyclase, although the enzyme systems responsible for this concerted redox reaction in humans remains the focus of very active investigation and continued controversy.
Several studies have investigated the possible enzymatic origins for mammalian nitrate and nitrite reduction. Most other genera of life, such as lower eukaryotes (e.g., plants and yeast) and prokaryotes (e.g., bacteria), possess efficient nitrate and nitrite reductase enzymes; however, mammals are believed to lack dedicated homologous enzymes. Still, a number of enzyme systems have been shown to control nitrite reduction to NO under certain conditions. As previously mentioned, xanthine oxidoreductase is a molybdenum-dependent enzymes that has been proposed as a mammalian nitrate and nitrite reductase.19, 20 Interestingly, xanthine oxidase exhibits a remarkable resemblance to the well-characterized plant nitrate reductases. Furthermore, a number of studies, including the current work by Baliga and colleagues, have clearly shown that the therapeutic effects of nitrite on PH require a functional xanthine oxidase system.21, 22 Interestingly, in human studies the inhibition of xanthine oxidase using oxypurinol does not inhibit nitrite-dependent blood flow, suggesting that xanthine oxidase does not mediate nitrite-dependent vasodilation in the human circulation.17
Several other mammalian nitrite reductase candidates have been proposed; these enzymes can be divided into groups based on active site metal content: molybdenum (xanthine oxidase and aldehyde oxidoreductase), iron (cytochrome c, eNOS, deoxyhemoglobin, deoxymyoglobin, neuroglobin), and copper (carbonic anhydrase).23-26 It is also possible that additional and potentially more effective (catalytic) human nitrate and nitrite reductases have not been identified.
Nitrite can be converted to nitric oxide by enzyme catalyzed oxidation-reduction (Scheme 1) or anhydrase (Scheme 2) reactions. The majority of nitrite reductase candidates are predicted to act as oxidoreductases (Scheme 1), while carbonic anhydrase has also been proposed to act as nitrite anhydrase. Note that hemoglobin may facilitate both reaction schemes.27 In Scheme 1, a simple electron and proton transfer reaction, the metal active site of the oxidoreductase enzyme can be oxidized while nitrite is reduced to nitric oxide. Two types of metal enzymes have been implicated in nitrite reduction to nitric oxide: molybdenum and iron. In Scheme 2, two nitrite molecules react to form dinitrogen trioxide (N2O3) and water, which then forms nitrite and NO spontaneously (non-enzymatic) by disproportionation.
Scheme 1.
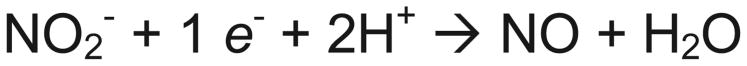
Scheme 2.
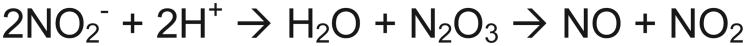
While the current study suggests that both nitrate and nitrite can prevent and reverse experimental PAH, there are a few caveats and an unexpected result. Firstly, the concentrations of both nitrate (45 mM) and nitrite (15 mM) in water are very high; by our estimates approximately 10 mg of nitrate or 3.5 mg of nitrite was ingested by the mice each day. A 70 kg human would have to ingest 36 grams of nitrate or 9 grams of nitrite each day to achieve these similar levels. Human research studies have been typically administered 500 to 1000 mg of nitrate each day, comparable to drinking about one liter of beet-root juice.18 Based on the Baliga study, one would have to drink 30 liters of beetroot juice each day to achieve similar results in humans with PAH. Moreover, these doses of nitrate and nitrite may generate significant levels of NO, nitrosothiols and nitrosamines in the stomach and will need to be carefully evaluated for safety and off-target effects.
The current study also demonstrated that allopurinol can prevent the beneficial effects of nitrite and nitrate on PAH, which is consistent with the work of other groups.21 However, the findings that the effects of nitrite are inhibited in eNOS null mice are unexpected and will require further study to understand. It is important to note that inhibition of eNOS does not block nitrite-vasodilatory effects in humans.7 Previous studies have shown that under anaerobic conditions endothelial NOS can reduce nitrite to NO; although, this is only effective under near-anaerobic conditions.28,29 One explanation is that oxygen tensions in the diseased lung are sufficiently low to allow nitrite reduction by eNOS, or there is an unexplained interaction in this system. Alternatively, we have reported that nitrite can also be oxidized by hydrogen peroxide or ferric hemoproteins to form oxidative signaling products, for example nitrogen dioxide. 30 It is possible that XO and eNOS in diseased vessels and tissues function not as nitrite reductases, but as nitrite oxidases via formation of superoxide and hydrogen peroxide. Further studies are required to address these important questions.
Acknowledgments
Dr. Gladwin receives research support from NIH grants R01HL098032, RO1HL096973, and PO1HL103455, the Institute for Transfusion Medicine and the Hemophilia Center of Western Pennsylvania.
Footnotes
Conflict of interest: Dr. Gladwin is listed as a co-inventor on an NIH government patent for the use of nitrite salts in cardiovascular diseases. Dr. Gladwin consults with Aires Pharmaceuticals on the development of a phase II proof of concept trial using inhaled nitrite for pulmonary arterial hypertension.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tabima DM, Frizzell S, Gladwin MT. Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radical Biology and Medicine. 2012 doi: 10.1016/j.freeradbiomed.2012.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 3.Zuckerbraun BS, Shiva S, Ifedigbo E, Mathier MA, Mollen KP, Rao J, Bauer PM, Choi JJW, Curtis E, Choi AMK, Gladwin MT. Nitrite potently inhibits hypoxic and inflammatory pulmonary arterial hypertension and smooth muscle proliferation via xanthine oxidoreductase–dependent nitric oxide generation. Circulation. 2010;121:98–109. doi: 10.1161/CIRCULATIONAHA.109.891077. [DOI] [PubMed] [Google Scholar]
- 4.Hunter CJ, Dejam A, Blood AB, Shields H, Kim-Shapiro DB, Machado RF, Tarekegn S, Mulla N, Hopper AO, Schechter AN, Power GG, Gladwin MT. Inhaled nebulized nitrite is a hypoxia-sensitive no-dependent selective pulmonary vasodilator. Nat Med. 2004;10:1122–1127. doi: 10.1038/nm1109. [DOI] [PubMed] [Google Scholar]
- 5.Gladwin MT, Shelhamer JH, Schechter AN, Pease-Fye ME, Waclawiw MA, Panza JA, Ognibene FP, Cannon RO. Role of circulating nitrite and s-nitrosohemoglobin in the regulation of regional blood flow in humans. Proceedings of the National Academy of Sciences; 2000; pp. 11482–11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modin A, Björne H, Herulf M, Alving K, Weitzberg E, Lundberg JON. Nitrite-derived nitric oxide: A possible mediator of ‘acidic–metabolic’ vasodilation. Acta Physiologica Scandinavica. 2001;171:9–16. doi: 10.1046/j.1365-201X.2001.00771.x. [DOI] [PubMed] [Google Scholar]
- 7.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 8.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Shiva S, Sack MN, Greer JJ, Duranski M, Ringwood LA, Burwell L, Wang X, MacArthur PH, Shoja A, Raghavachari N, Calvert JW, Brookes PS, Lefer DJ, Gladwin MT. Nitrite augments tolerance to ischemia/reperfusion injury via the modulation of mitochondrial electron transfer. The Journal of Experimental Medicine. 2007;204:2089–2102. doi: 10.1084/jem.20070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin N, O'Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach no synthesis. Nature. 1994;368:502–502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 11.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Björne H, Petersson J, Phillipson M, Weitzberg E, Holm L, Lundberg JO. Nitrite in saliva increases gastric mucosal blood flow and mucus thickness. J Clin Invest. 2004;113:106–114. doi: 10.1172/JCI200419019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pankey EA, Badejo AM, Casey DB, Lasker GF, Riehl RA, Murthy SN, Nossaman BD, Kadowitz PJ. Effect of chronic sodium nitrite therapy on monocrotaline-induced pulmonary hypertension. Nitric Oxide. 2012 doi: 10.1016/j.niox.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casey DB, Badejo AM, Dhaliwal JS, Murthy SN, Hyman AL, Nossaman BD, Kadowitz PJ. Pulmonary vasodilator responses to sodium nitrite are mediated by an allopurinol-sensitive mechanism in the rat. American Journal of Physiology - Heart and Circulatory Physiology. 2009;296:H524–H533. doi: 10.1152/ajpheart.00543.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lundberg JO, Weitzberg E, Lundberg JE, Alving K. Intragastric nitric oxide production in humans: Measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govoni M, Jansson EA, Weitzberg E, Lundberg JO. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide. 2008;19:333–337. doi: 10.1016/j.niox.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 18.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Letters. 1998;427:225–228. doi: 10.1016/s0014-5793(98)00430-x. [DOI] [PubMed] [Google Scholar]
- 20.Jansson EA, Huang L, Malkey R, Govoni M, Nihlen C, Olsson A, Stensdotter M, Petersson J, Holm L, Weitzberg E, Lundberg JO. A mammalian functional nitrate reductase that regulates nitrite and nitric oxide homeostasis. Nat Chem Biol. 2008;4:411–417. doi: 10.1038/nchembio.92. [DOI] [PubMed] [Google Scholar]
- 21.Zuckerbraun BS, George P, Gladwin MT. Nitrite in pulmonary arterial hypertension: Therapeutic avenues in the setting of dysregulated arginine/nitric oxide synthase signalling. Cardiovascular Research. 2011;89:542–552. doi: 10.1093/cvr/cvq370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alef MJ, Vallabhaneni R, Carchman E, Morris SM, Shiva S, Wang Y, Kelley EE, Tarpey MM, Gladwin MT, Tzeng E, Zuckerbraun BS. Nitrite-generated no circumvents dysregulated arginine/nos signaling to protect against intimal hyperplasia in sprague-dawley rats. The Journal of Clinical Investigation. 2011;121:1646–1656. doi: 10.1172/JCI44079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood. 2008;112:2636–2647. doi: 10.1182/blood-2008-01-115261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: A possible link between metabolic activity and vasodilation. American Journal of Physiology - Heart and Circulatory Physiology. 2009;297:H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 25.Shiva S, Huang Z, Grubina R, Sun J, Ringwood LA, MacArthur PH, Xu X, Murphy E, Darley-Usmar VM, Gladwin MT. Deoxymyoglobin is a nitrite reductase that generates nitric oxide and regulates mitochondrial respiration. Circulation Research. 2007;100:654–661. doi: 10.1161/01.RES.0000260171.52224.6b. [DOI] [PubMed] [Google Scholar]
- 26.Jayaraman T, Tejero J, Chen BB, Blood AB, Frizzell S, Shapiro C, Tiso M, Hood BL, Wang X, Zhao X, Conrads TP, Mallampalli RK, Gladwin MT. 14-3-3 binding and phosphorylation of neuroglobin during hypoxia modulate six-to-five heme pocket coordination and rate of nitrite reduction to nitric oxide. Journal of Biological Chemistry. 2011;286:42679–42689. doi: 10.1074/jbc.M111.271973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of n2o3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat Chem Biol. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 28.Gautier C, van Faassen E, Mikula I, Martasek P, Slama-Schwok A. Endothelial nitric oxide synthase reduces nitrite anions to no under anoxia. Biochemical and Biophysical Research Communications. 2006;341:816–821. doi: 10.1016/j.bbrc.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Vanin AF, Bevers LM, Slama-Schwok A, Van Faassen EE. Nitric oxide synthase reduces nitrite to no under anoxia. Cellular and Molecular Life Sciences. 2007;64:96–103. doi: 10.1007/s00018-006-6374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Frizzell SA, Zhao X, Gladwin MT. Normoxic cyclic gmp-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing. Nitric Oxide. doi: 10.1016/j.niox.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


