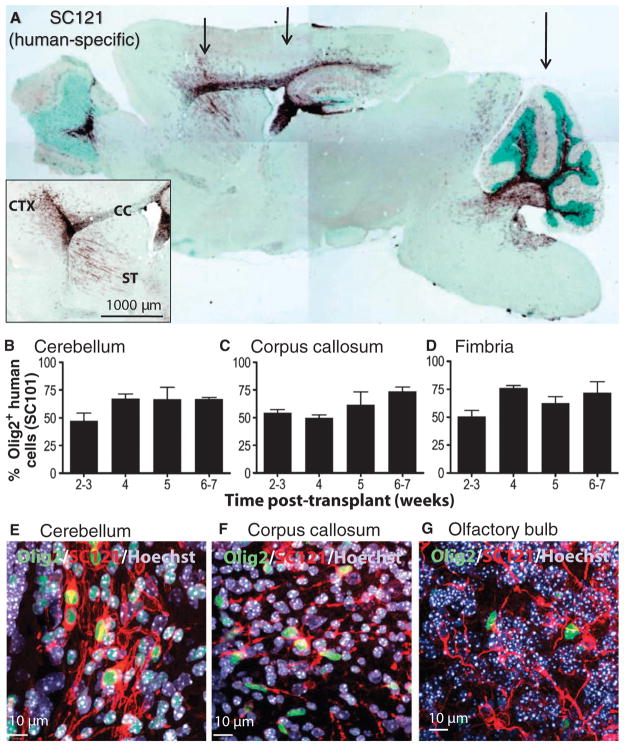
Fig. 1.
Migration and differentiation of HuCNS-SCs after transplantation into neonatal Shi-id mouse. (A) Cells were transplanted bilaterally at three coronal levels (arrows) that targeted the forebrain areas (striatum, rostral corpus callosum, and anterior cortex), the midbrain areas (hippocampus, body of the corpus callosum, and posterior cortex), and the hindbrain areas (cerebellum and brainstem). SC121 staining (brown) at 8 weeks after transplant demonstrated extensive migration of HuCNS-SCs within white matter tracts that included the corpus callosum, fimbria of the fornix, and those in the cerebellum. (Inset) Detail of SC121 staining from an adjacent section from the same animal revealed robust engraftment at the injection core and migration of the HuCNS-SC progeny into the cortex (CTX), corpus callosum (CC), and striatum (ST) 8 weeks after transplant. Regional boundaries are delineated with methyl green counter-stain. (B to D) Quantification of the percentage of human cells committed to the oligodendrocyte lineage at successive time points after transplantation. Human oligodendrocytes were identified by coexpression of the human nuclear marker SC101+ and the nuclear transcription factor Olig2 in the cerebellum (B), corpus callosum (C), and fimbria (D). (E to G) Immunofluorescent staining for Olig2 (green), human-specific cytoplasmic marker SC121 (red), and Hoechst 33324 (nuclear stain, blue) in cerebellum (E), corpus callosum (F), and olfactory bulb (G).