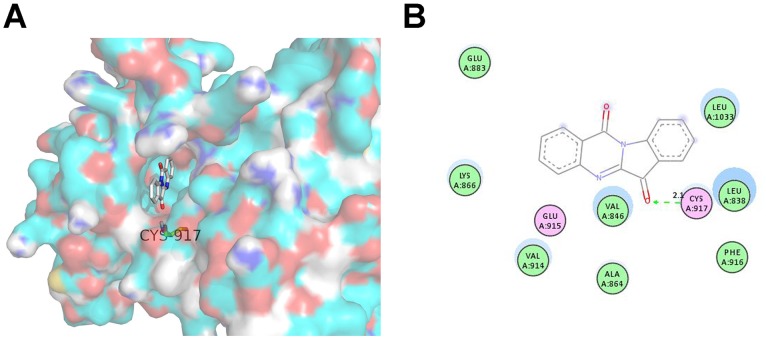
Figure 8. The favourable binding position of tryptanthrin with lowest binding free energy in the ATP-binding site of VEGFR2 (PDB code 1YWN) as analyzed by molecular docking study.
(A) The three-dimensional diagram displays the interaction of tryptanthrin (the white stick) to the ATP-binding site of VEGFR2 with the labelled amino acid residue Cys917 which significantly contributed to the binding. (B) The two-dimensional diagram shows the interactions of tryptanthrin to the amino acid residues in the ATP-binding site. Colors of the residues indicate the forms of interactions as follows: van der Waals forces, green; polarity, magenta. Green arrow represents H-bonding with the amino acid main chain.