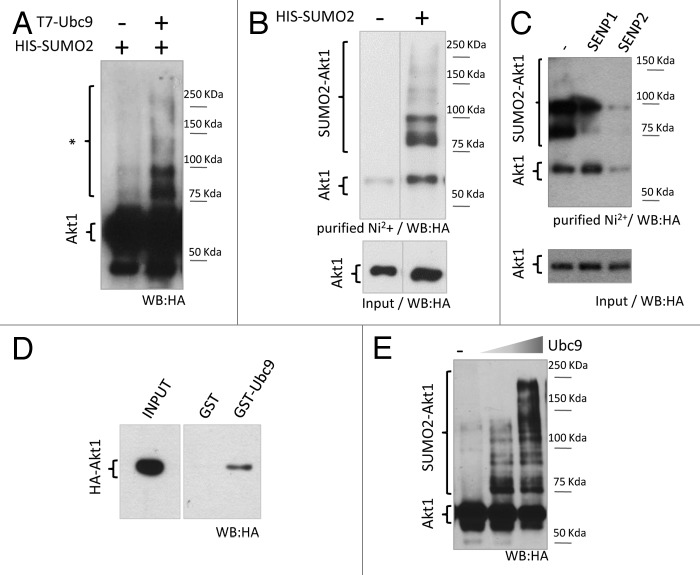
Figure 1. Akt1 is a SUMO substrate in cells and in vitro. (A) HEK 293T cells were co-transfected with expression vectors for HA-tagged Akt1 and His-SUMO2, with or without T7-Ubc9 expression vector. Cell lysates were prepared 48 h after transfection and analyzed by SDS/page and western blot with an anti-HA antibody. Slower migrating bands suspected to be SUMO-Akt1 conjugates are indicated with an asterisk. (B) HEK 293T cells were transfected with HA-Akt1 and Ubc9 expression vectors, with or without His-SUMO2. After 48 h, cells were lysed, an aliquot of the lysate was taken as input, and the reminder was subject to denaturing Ni2+ affinity chromatography. Both fractions were analyzed by western blot with an anti-HA antibody. Different lanes from the same gel were put together as indicated by the dividing line. (C) Cells were co-transfected with HA-Akt1, His-SUMO2, Ubc9 and either SENP1 or 2 expression vectors, or the corresponding empty vector (−). SUMO conjugation to Akt1 was analyzed as in (B). (D) Pull-down assay (right panel) was performed combining bacterially expressed and purified GST or GST-Ubc9 with cell lysates from HEK 293T expressing HA-Akt1. An aliquot of the lysate was taken as input (left panel). Samples were analyzed by western blot with an anti-HA antibody. (E) Purified recombinant HA-Akt1 was incubated with Aos1-Uba2 (E1 heterodimer), increasing amounts of Ubc9 (E2 enzyme), and SUMO2. Reactions were stopped by addition of 2X Laemmli sample buffer and analyzed by western blot with an anti-HA antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.