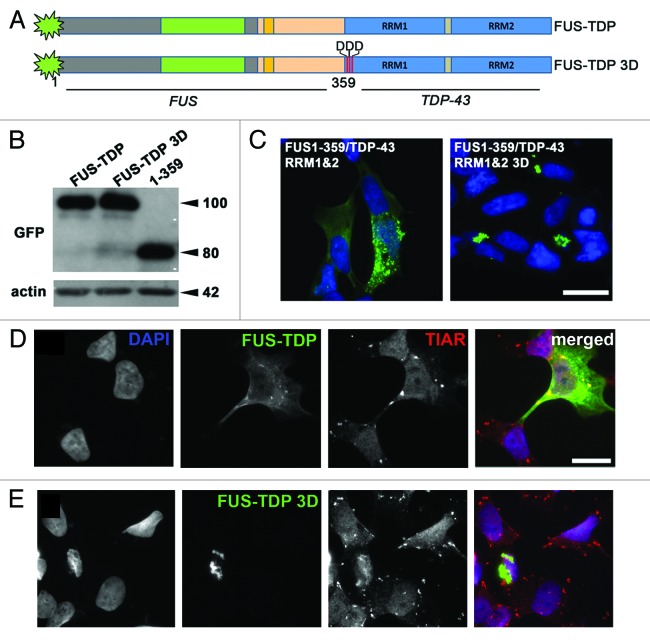
Figure 5. Aggregation of truncated FUS 1–359 in cultured cells can be prevented by fusion with functional RNA-binding domains of TDP-43. (A) Scheme of FUS-TDP-43 chimeric proteins. (B) Western blot analysis of FUS 1–359 and FUS-TDP-43 fusions using anti-GFP antibody demonstrates their equal levels upon expression in neuroblastoma SH-SY5Y cells. The size of proteins in kDa is shown. (C) Intracellular distribution of GFP-tagged chimeric proteins in transfected SH-SY5Y cells reveals granule-like aggregates with diffuse distribution of FUS-TDP-43 with intact RRMs and formation of aggresomes when RNA-binding capacity of RRM1 is abolished by 3 amino acid substitutions, L106D, V108D, L111D (3D mutant). Scale bar: 15 µm for both panels. (D and E) FUS-TDP-43 with intact RRMs (D) but not FUS-TDP-43 bearing substitutions in RRM1 disrupting RNA binding (E) is able to enter stress granules induced by arsenite treatment. Scale bar: 15 µm for all panels.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.