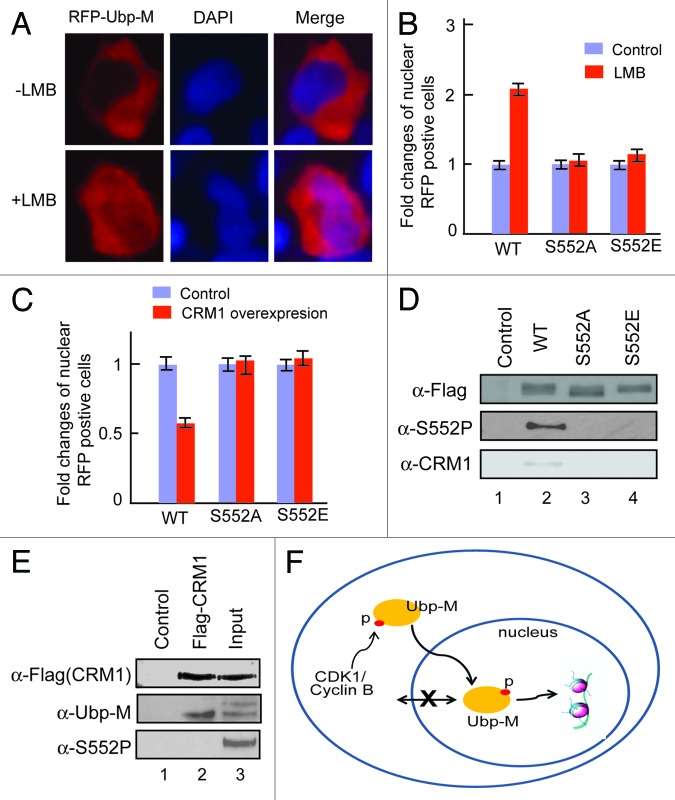
Figure 5.
Ubp-M S552P regulates the interaction between Ubp-M and CRM1. (A) Ubp-M subcellular localization is regulated by CRM1 mediated nuclear export. LMB treatment increased the nuclear localization of RFP-Ubp-M. Two hundred RFP-positive cells were examined and representative images shown. (B) Fold change in the number of cells with nuclear wild-type or mutant (S552A or S552E) RFP-Ubp-M in control and LMB treated cells. 200 RFP-positive cells were examined. The percentage of cells with nuclear wild-type, S552A or S552E RFP-Ubp-M without LMB treatment was set to 1. (C) Fold change of nuclear wild-type and mutant (S552A and S552E) RFP-Ubp-M in control and CRM1-overexpressing cells. 200 RFP-positive cells were examined. The percentage of nuclear wild-type and mutant (S552A and S552E) Ubp-M in control cells was set to 1. (D) Ubp-M S552P regulates the interaction between Ubp-M and CRM1. CRM1 was only detected in anti-Flag immunoprecipitates of wild-type, but not S552A and S552E mutant, Ubp-M. (E) CRM1 interacts with non-phosphorylated Ubp-M. CRM1 specifically pulled down non-phosphorylated Ubp-M but not phosphorylated Ubp-M, as confirmed by anti-S552P antibody. (F) A proposed model for the function of Ubp-M S552P. Ubp-M subcellular localization is determined by the balance between export and import processes. As cells prepare to enter M phase, cytoplasmic cycle B/CDK1 kinase is activated and phosphorylates Ubp-M S552. S552P disrupts Ubp-M interaction with CRM1, allowing Ubp-M to be retained in the nucleus and deubiquitinate nucleosomal uH2A, which is required for chromosome condensation and cell cycle progression.