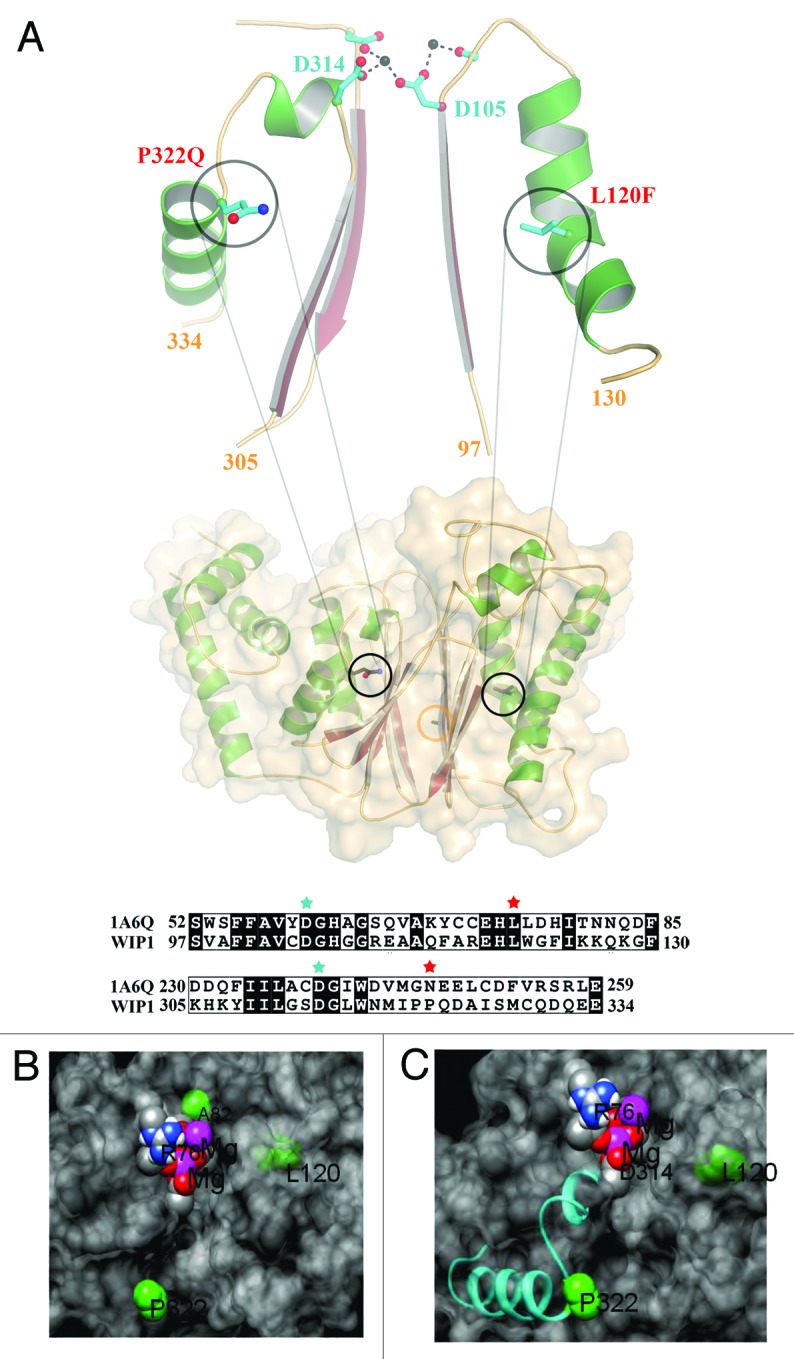
Figure 1. The structural model for Wip1 genetic variants. (A) The model of Wip1 was generated based on the structure of phosphatase 2C (1A6G). L120F and P322Q point towards β-sheets in this α-β-β-α sandwich fold. Both mutations are located in α-helices that follow loops, which contain metal ion coordinating aspartic acids in the active site, Asp105 and Asp314, respectively. The L120F (L is shown) mutation may be expected to alter the conformation of the preceding loop and Asp105 due to its increased size being accommodated in the α-β packing. P322Q (Q is shown) defines the end of the α-helix. The restricted geometry of helix-breaking proline and the increased bulk of glutamine will similarly affect the path of the preceding loop and Asp314. Sequence alignments of these regions of Wip1 with phosphatase 2C (1A6G) are shown in the lower panel. L120 and P322 are highlighted by red stars. D105 and D314 are denoted by cyan stars. The position of Ala82 is demarked by an orange circle. The model was generated in the program COOT. (B and C) Close views of the variants (green) in relation to the surface (gray), magnesium ions (purple), oxygen atoms of D105 and D314 (red) and the P332 containing helix (cyan).
