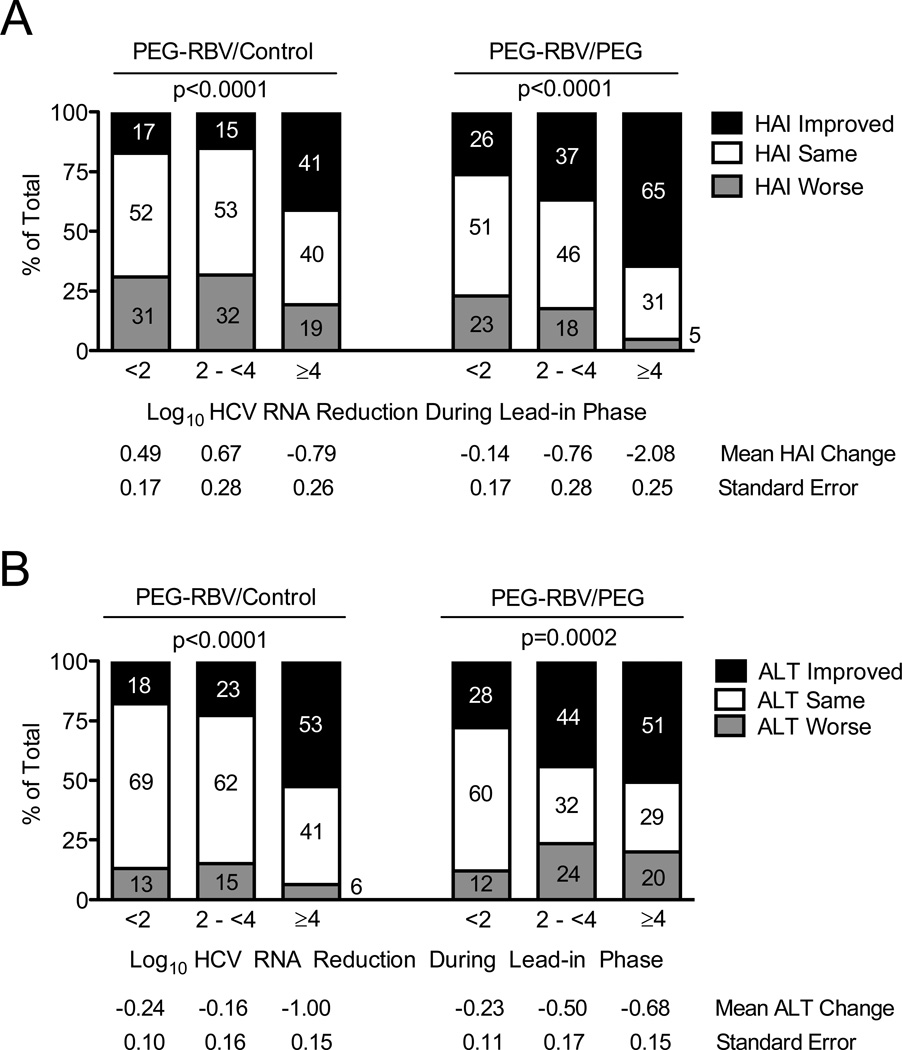
Figure 2. HCV RNA Suppression During Lead-in Therapy is Associated with Improvement in Hepatic Inflammation and ALT During the Randomized Phase.
Patients were categorized according to their randomized untreated control (left) or treated status (right) and their degree of HCV RNA suppression during the lead-in phase of the HALT-C Trial (x-axis): <2 log10, 2-<4 log10, and ≥4 log10 decrease between baseline and week 20. Percentages of patients with improvement or worsening of hepatic inflammation by ≥2 points or with no change between baseline and year-1.5 biopsies are indicated on the y-axis and by the numbers in the columns (A). The mean change in HAI and associated standard error for the group of patients represented by each column is shown below the x-axis. The percentage of patients with ALT improvement (ALT decrease by >25%), ALT worsening (ALT increase by >25%) and no change (ALT change ≤25%) between baseline and year 1.5 are shown on the y-axis and by the numbers in the columns (B). The mean change in ALT and associated standard error for the group of patients represented by each column is shown below the x-axis. Percent ALT change was calculated as described for Figure 1. P values are based on a chi-square test for trend in the % improved. The number of patients represented by each column are as follows: PEG-RBV/Control groups <2 log10 reduction n = 185, 2 to <4 log10 reduction n = 66, ≥4 log10 reduction n = 78; PEG-RBV/PEG groups <2 log10 reduction n = 175, 2 to <4 log10 reduction n = 68, ≥4 log10 reduction n = 85.