Abstract
Fiber tracking provides insights into the brain white matter network and has become more and more popular in diffusion MR imaging. Hardware or software phantom provides an essential platform to investigate, validate and compare various tractography algorithms towards a “gold standard”. Software phantoms excel due to their flexibility in varying imaging parameters, such as tissue composition, SNR, as well as potential to model various anatomies and pathologies. This paper describes a novel method in generating diffusion MR images with various imaging parameters from realistically appearing, individually varying brain anatomy based on predefined fiber tracts within a high-resolution human brain atlas. Specifically, joint, high resolution DWI and structural MRI brain atlases were constructed with images acquired from 6 healthy subjects (age 22–26) for the DWI data and 56 healthy subject (age 18–59) for the structural MRI data. Full brain fiber tracking was performed with filtered, two-tensor tractography in atlas space. A deformation field based principal component model from the structural MRI as well as unbiased atlas building was then employed to generate synthetic structural brain MR images that are individually varying. Atlas fiber tracts were accordingly warped into each synthetic brain anatomy. Diffusion MR images were finally computed from these warped tracts via a composite hindered and restricted model of diffusion with various imaging parameters for gradient directions, image resolution and SNR. Furthermore, an open-source program was developed to evaluate the fiber tracking results both qualitatively and quantitatively based on various similarity measures.
Keywords: Diffusion-weighted MRI, Atlas, Fiber tracking, Tractography, Phantom, Validation
1. INTRODUCTION
Fiber tracking has become more and more popular in diffusion MR imaging as it provides unique insights into the brain white matter network. Hardware or software phantom serve as an essential platform to investigate, validate and compare various tractography algorithms towards a “gold standard”. Software phantoms excel due to their flexibility in varying imaging parameters, such as tissue composition, SNR, as well as potential to model various anatomies and pathologies. This paper describes a novel method in generating diffusion MR phantoms with various imaging parameters from realistically appearing, individually varying brain anatomy based on predefined fiber tracts within an “arbitrary” high-resolution human brain atlas. The main objective of this study is to provide an essential software tool to generate human brain diffusion MR phantoms from any “ground truth” fiber tracts, that could easily adapt to varying anatomy and imaging parameters. They serve as crucial platforms to qualitatively and quantitatively evaluate various fiber tracking techniques.
2. METHOD
2.1 Creation of joint high resolution atlas with fiber tracts
All MR images used for atlas computation were acquired on a Siemens 3T scanner. Images include T1 and T2 acquired at 1×1×1 mm3 and diffusion weighted (DW) images along 42 unique directions at b=1000 s/mm2 and 7 baseline images at b=0 (1.5×1.5×1.5 mm3).
The structural atlas was determined from a set of 56 training images (subjects with age ranging 20–561) by iterative joint deformable registration of all training datasets into a single unbiased average image that has minimal deformation to all training images, as described in.2 Prior to the deformable atlas building step, the training images were affinely registered, skull stripped and intensity calibrated via pairwise histogram quantile matching. All the training MR brain images were from healthy volunteers collected and made available by the CASILab at The University of North Carolina at Chapel Hill and were distributed by the MIDAS Data Server at Kitware, Inc.
The DW atlas consisted of 6 healthy subjects age ranging from 22 to 26. Diffusion tensors were computed using weighted least squares fitting3 via the NA-MIC DTIProcess software suite. Eigenvalues and corresponding eigenvectors were calculated yielding the fractional anisotropy (FA) maps. The same algorithm used for the structural atlas computation was also used to build an unbiased atlas of FA yielding diffeomorphic field maps that warp each individual subject to the atlas. Corresponding DWIs were then transformed into the atlas space via a robust parametric model-based estimation method.4 The DWI atlas was computed using the weighted least square (WLS) method4 with an 8th order symmetric spherical harmonics (SH) basis.
The structural atlas was subsequently transformed into the DW atlas space by first registering the T2 atlas to the baseline (b=0) image of the DW atlas via b-spline registration5 followed by application of this transformation on both T1 and T2 atlas.
Full brain fiber tracking was done on the DWI atlas by performing filtered two-tensor tractography6 on the whole brain white matter. Specifically, a brain white matter (WM) mask was generated by a simple thresholding of FA ≥ 0.2. Fiber tracts were traced starting from 64 seed points per imaging voxel on the WM mask to their termination using an unscented Kalman filter to simultaneously fit the signal using a two-tensor model and propagate in the most consistent direction with prior knowledge.6
2.2 Creation of DW phantoms in varying synthetic human brain anatomy
Synthetic varying phantoms were created by applying “arbitrary” transformations that were generated as deformation fields to the co-registered atlas (T1/T2 and DW). An orthogonal basis representative of the whole training population was generated by performing principal component analysis (PCA) on the deformation fields that warped training images to the atlas. Subsequently, 19 “arbitrary” deformations, similar to the ones in the training set were constructed as linear combinations of the principal components. Reverse transformation that warped the corresponding synthetic phantom to the atlas space was accordingly applied to the full brain fiber tracts yielding full brain tracts in 19 varying individual anatomy space as shown in Fig. 2.1.
Figure 2.
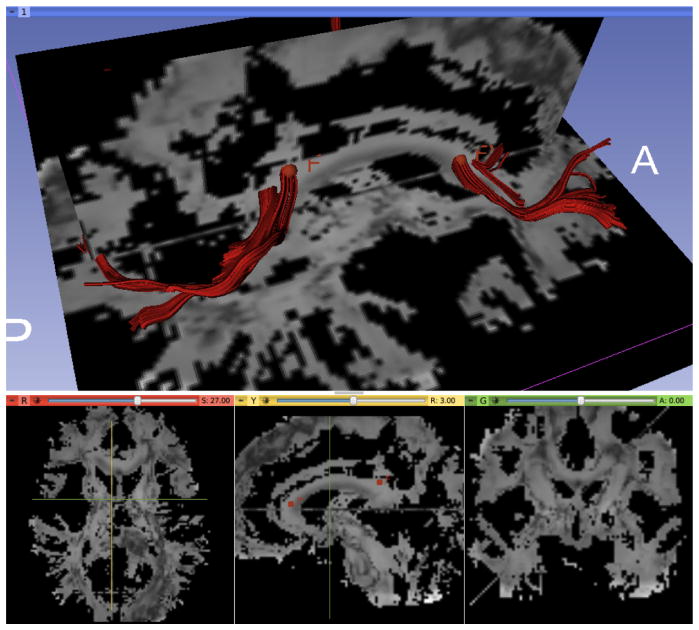
FA with simple fiducial-based fiber tracking of the genu and splenium fibers using Slicer4
Diffusion signal was estimated via composite hindered and restricted model (CHARMED)7 on a voxel-to-voxel base. In a nutshell, for each voxel, DW signal was modeled as hindered and restricted components, where the hindered component represents the background diffusion and the restricted ones characterizing all the fibers passing through this voxel. Rician noise was added to the DW signal afterwards. Mathematically, it could be described as , where fr and fh are the volume fractions of the hindered and restricted compartments and follow that fr + fh = 1; Eh(q, Δ) and Er(q?, Δ) are the normalized MR echo signals from the hindered and restricted compartments; i denotes the ith fiber tract; N represents added noise.
2.3 Evaluation software for fiber tracking results
Open-source software (soon to be available on NITRC8), comparing statistically any given tractography result against the “ground truth” was developed for evaluation purposes. Four statistical measures were included inspired by.9 Specifically, fiber tracts were first re-sampled in the same fashion by modeling them as parametrized b-splines. Different metrics were then implemented to compare the given tractography result with the “ground truth”, including point-wise measures along the fibers: 1)spatial metric, which computes the L2 norm of the fiber orientations at corresponding positions, 2)curve metric, which computes the absolute difference of curvatures between the corresponding fiber points and volumetric measures: 3)absolute volume overlap and 4)probabilistic volume overlap, where fiber tracts were first “voxelized” as images. Binary images were created for absolute volume overlap measure (1 if the fiber passes through a given voxel, 0 otherwise) while grayscale images were created for probabilistic volume overlap measure, where the intensity denotes the number of fibers passing through a given voxel. Similarity measures, including absolute geometric overlap, mean distance and Hausdorff distance between the two binary/grayscale images generated from the “ground truth” and the testing tractography method were computed.
3. RESULTS
DW phantoms were generated in 19 varying anatomy space. Fiber tracking was performed for validation. FA of DW phantom using one set of imaging parameters were shown in Fig. 4 with genu and splenium traced with Slicer4 (www.slicer.org). Experiments with different imaging parameters, including TE/TR and diffusion encoding gradients for the scanning sequence, SNR, diffusivity and coefficients for hindered and restricted compartments respectively7 were performed for DW phantom generation (figures not shown due to space limit).
4. CONCLUSIONS
We developed a novel method that generates DW software phantoms given any “ground truth” fiber tracts and desired brain anatomy as well as a set of evaluation methods to compare the fiber tracking results with the “ground truth”. To our knowledge, this is the first time a complete software DW phantom generation pipeline was developed that could model various human anatomy including various pathologies. In this paper, we implemented 19 DW phantoms via CHARMED model in varying brain anatomy (to be published as testing dataset for the tractography challenge workshop (dti-challenge.org) and tested several fiber tracking algorithms as well as compared them quantitatively using our tractography evaluation software. It is noteworthy that the method is not limited to the fiber tracking algorithm generating the pre-defined tracts and could easily adapt to simulate diffusion signal besides the employed CHARMED model. All the programs will soon be available on NITRC.8
Figure 1.
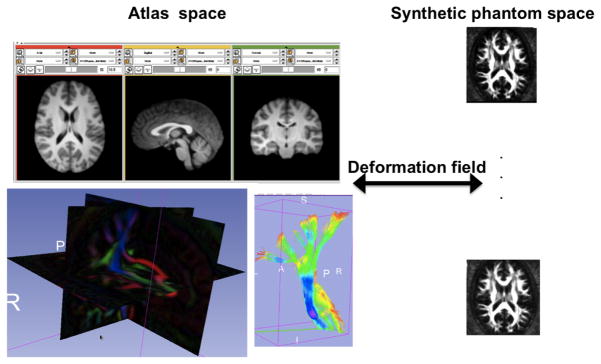
Ground truth fiber tracts in the atlas space that were pre-defined were transformed by a deformation field to any given phantom space. Here, the T1 atlas was co-registered to the DWI atlas space and fiber tracts from the DWI atlas were transformed into synthetic phantom space that were generated with PCA analysis arbitrarily.
References
- 1.EB, et al. Vessel tortuosity and brain tumor malignancy: a blinded study. Acad Radiol. 2005;12(10):1232–40. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.SJ, et al. Unbiased diffeomorphic atlas construction for computational anatomy. Neuro Image. 2004;23:S151–S160. doi: 10.1016/j.neuroimage.2004.07.068. [DOI] [PubMed] [Google Scholar]
- 3.CG, et al. Group analysis of dti fiber tract statistics with application to neurodevelopment. Neuro Image. 2009;45(1, Supplement 1):S133– S142. doi: 10.1016/j.neuroimage.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MN, et al. MICCAI CDMRI’10. 2010. Robust model-based transformation and averaging of diffusion weighted images – applied to diffusion weighted atlas construction. [Google Scholar]
- 5.DR, et al. Diffeomorphic registration using b-splines. MICCAI. 2006 Jan;:702–9. doi: 10.1007/11866763_86. [DOI] [PubMed] [Google Scholar]
- 6.JM, et al. A filtered approach to neural tractography using the watson directional function. Medical Image Analysis. 2010;14(1):58– 69. doi: 10.1016/j.media.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaf Y, Basser P. Composite hindered and restricted model of diffusion (charmed) mr imaging of the human brain. Neuro Image. 2005;27(1):48– 58. doi: 10.1016/j.neuroimage.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 8.NP, et al. Neuroimaging informatics tools and resources clearinghouse (nitrc) 2011 May; http://www.nitrc.org. [PubMed]
- 9.PF, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion mr phantom. Neuroimage. 2011;56(1):220–34. doi: 10.1016/j.neuroimage.2011.01.032. [DOI] [PubMed] [Google Scholar]