Abstract
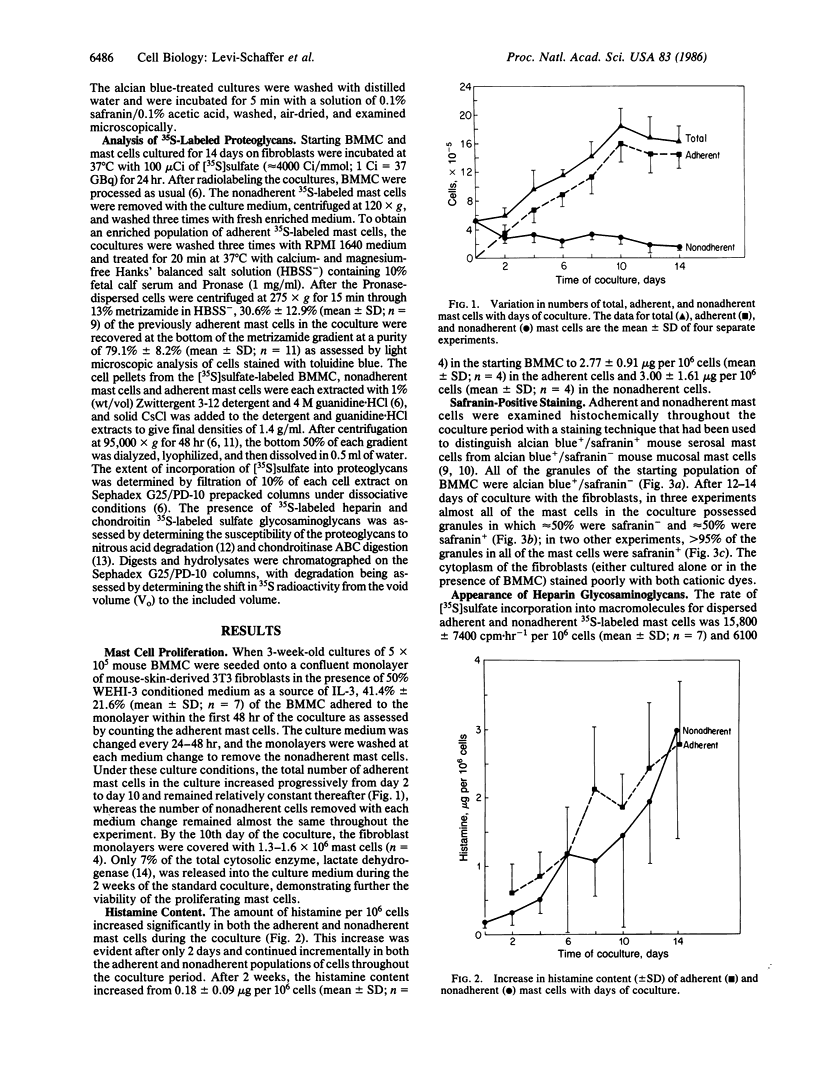
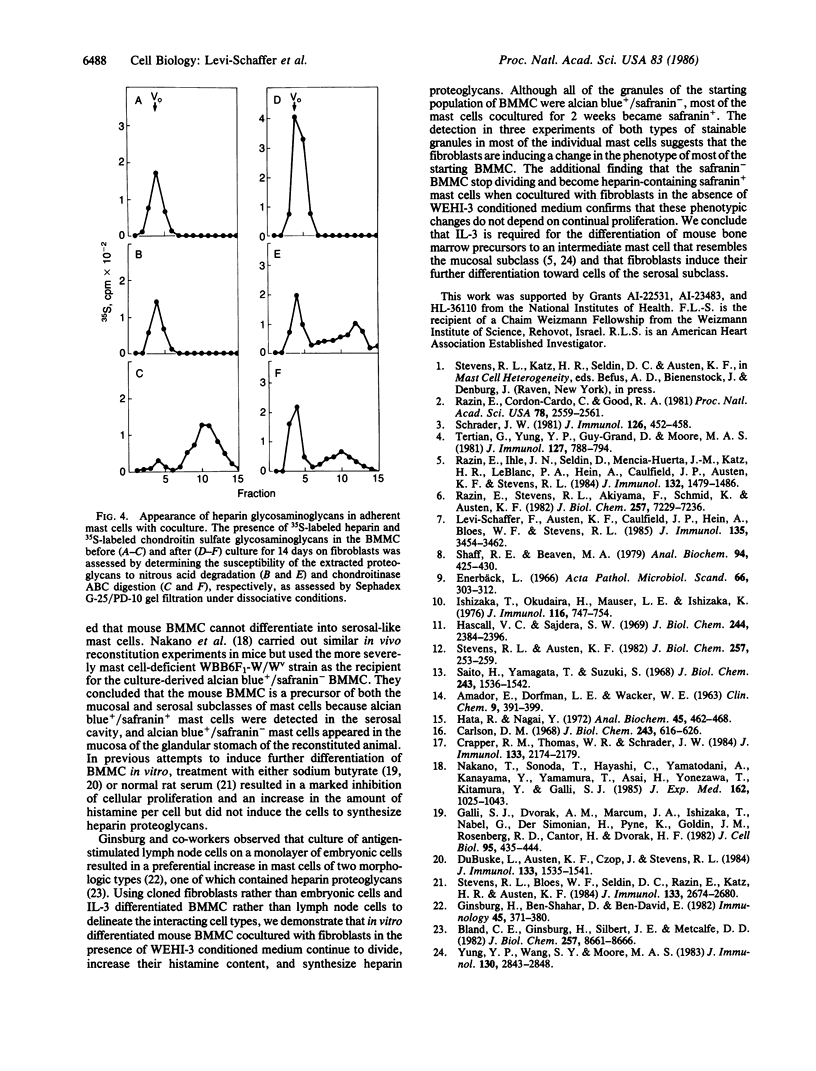
The heparin-containing mast cells that reside in the connective tissue of the mouse, but not the chondroitin sulfate-containing mast cells in the gastrointestinal mucosa, stain with safranin when exposed to alcian blue/safranin. Mouse bone marrow-derived mast cells (BMMC), the probable in vitro counterparts of in vivo mucosal mast cells, were cultured for 14 days with mouse skin-derived 3T3 fibroblasts in RPMI 1640 medium containing 10% fetal calf serum and 50% WEHI-3 conditioned medium. Although the BMMC adhered to the fibroblast monolayer, they continued to divide, probably due to the presence of interleukin 3 in the conditioned medium. The mast cells remained viable throughout the period of coculture, since they failed to release lactate dehydrogenase and because they increased their histamine content approximately 15-fold. After 12-14 days of coculture, greater than 50% of the BMMC changed histochemically to become safranin+; 30-40% of the 35S-labeled glycosaminoglycans on the proteoglycans synthesized by these cocultured mast cells were heparin, whereas heparin was not detected in the initial BMMC. In the absence of WEHI-3 conditioned medium, BMMC adhered to the fibroblast monolayer, and after 8 days of coculture, the number of mast cells did not change and their histamine content remained the same. However, these mast cells also became safranin+ and synthesized 40% heparin glycosaminoglycans. Thus, coculture of BMMC with fibroblasts induces a phenotypic change so that the resulting mast cells stain safranin+ and synthesize heparin proteoglycans, whereas the presence of WEHI-3 conditioned medium stimulates proliferation and an increase in histamine content.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMADOR E., DORFMAN L. E., WACKER W. E. SERUM LACTIC DEHYDROGENASE ACTIVITY: AN ANALYTICAL ASSESSMENT OF CURRENT ASSAYS. Clin Chem. 1963 Aug;12:391–399. [PubMed] [Google Scholar]
- Bland C. E., Ginsburg H., Silbert J. E., Metcalfe D. D. Mouse heparin proteoglycan. Synthesis by mast cell-fibroblast monolayers during lymphocyte-dependent mast cell proliferation. J Biol Chem. 1982 Aug 10;257(15):8661–8666. [PubMed] [Google Scholar]
- Carlson D. M. Structures and immunochemical properties of oligosaccharides isolated from pig submaxillary mucins. J Biol Chem. 1968 Feb 10;243(3):616–626. [PubMed] [Google Scholar]
- Crapper R. M., Thomas W. R., Schrader J. W. In vivo transfer of persisting (P) cells; further evidence for their identity with T-dependent mast cells. J Immunol. 1984 Oct;133(4):2174–2179. [PubMed] [Google Scholar]
- DuBuske L., Austen K. F., Czop J., Stevens R. L. Granule-associated serine neutral proteases of the mouse bone marrow-derived mast cell that degrade fibronectin: their increase after sodium butyrate treatment of the cells. J Immunol. 1984 Sep;133(3):1535–1541. [PubMed] [Google Scholar]
- Enerbäck L. Mast cells in rat gastrointestinal mucosa. 2. Dye-binding and metachromatic properties. Acta Pathol Microbiol Scand. 1966;66(3):303–312. doi: 10.1111/apm.1966.66.3.303. [DOI] [PubMed] [Google Scholar]
- Galli S. J., Dvorak A. M., Marcum J. A., Ishizaka T., Nabel G., Der Simonian H., Pyne K., Goldin J. M., Rosenberg R. D., Cantor H. Mast cell clones: a model for the analysis of cellular maturation. J Cell Biol. 1982 Nov;95(2 Pt 1):435–444. doi: 10.1083/jcb.95.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Ben-Shahar D., Ben-David E. Mast cell growth on fibroblast monolayers: two-cell entities. Immunology. 1982 Feb;45(2):371–380. [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hata R., Nagai Y. A rapid and micro method for separation of acidic glycosaminoglycans by two-dimensional electrophoresis. Anal Biochem. 1972 Feb;45(2):462–468. doi: 10.1016/0003-2697(72)90208-4. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Okudaira H., Mauser L. E., Ishizaka K. Development of rat mast cells in vitro. I. Differentiation of mast cells from thymus cells. J Immunol. 1976 Mar;116(3):747–754. [PubMed] [Google Scholar]
- Levi-Schaffer F., Austen K. F., Caulfield J. P., Hein A., Bloes W. F., Stevens R. L. Fibroblasts maintain the phenotype and viability of the rat heparin-containing mast cell in vitro. J Immunol. 1985 Nov;135(5):3454–3462. [PubMed] [Google Scholar]
- Nakano T., Sonoda T., Hayashi C., Yamatodani A., Kanayama Y., Yamamura T., Asai H., Yonezawa T., Kitamura Y., Galli S. J. Fate of bone marrow-derived cultured mast cells after intracutaneous, intraperitoneal, and intravenous transfer into genetically mast cell-deficient W/Wv mice. Evidence that cultured mast cells can give rise to both connective tissue type and mucosal mast cells. J Exp Med. 1985 Sep 1;162(3):1025–1043. doi: 10.1084/jem.162.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Cordon-Cardo C., Good R. A. Growth of a pure population of mouse mast cells in vitro with conditioned medium derived from concanavalin A-stimulated splenocytes. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2559–2561. doi: 10.1073/pnas.78.4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin E., Ihle J. N., Seldin D., Mencia-Huerta J. M., Katz H. R., LeBlanc P. A., Hein A., Caulfield J. P., Austen K. F., Stevens R. L. Interleukin 3: A differentiation and growth factor for the mouse mast cell that contains chondroitin sulfate E proteoglycan. J Immunol. 1984 Mar;132(3):1479–1486. [PubMed] [Google Scholar]
- Razin E., Stevens R. L., Akiyama F., Schmid K., Austen K. F. Culture from mouse bone marrow of a subclass of mast cells possessing a distinct chondroitin sulfate proteoglycan with glycosaminoglycans rich in N-acetylgalactosamine-4,6-disulfate. J Biol Chem. 1982 Jun 25;257(12):7229–7236. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Schrader J. W. In in vitro production and cloning of the P cell, a bone marrow-derived null cell that expresses H-2 and Ia-antigens, has mast cell-like granules, and is regulated by a factor released by activated T cells. J Immunol. 1981 Feb;126(2):452–458. [PubMed] [Google Scholar]
- Shaff R. E., Beaven M. A. Increased sensitivity of the enzymatic isotopic assay of histamine: measurement of histamine in plasma and serum. Anal Biochem. 1979 Apr 15;94(2):425–430. doi: 10.1016/0003-2697(79)90385-3. [DOI] [PubMed] [Google Scholar]
- Stevens R. L., Austen K. F. Effect of p-nitrophenyl-beta-D-xyloside on proteoglycan and glycosaminoglycan biosynthesis in rat serosal mast cell cultures. J Biol Chem. 1982 Jan 10;257(1):253–259. [PubMed] [Google Scholar]
- Stevens R. L., Bloes W. F., Seldin D. C., Razin E., Katz H. R., Austen K. F. Inhibition of proliferation of mouse T cell-dependent bone marrow-derived mast cells by rat serum does not change their unique phenotype. J Immunol. 1984 Nov;133(5):2674–2680. [PubMed] [Google Scholar]
- Tertian G., Yung Y. P., Guy-Grand D., Moore M. A. Long-term in vitro culture of murine mast cells. I. Description of a growth factor-dependent culture technique. J Immunol. 1981 Aug;127(2):788–794. [PubMed] [Google Scholar]
- Yung Y. P., Wang S. Y., Moore M. A. Characterization of mast cell precursors by physical means: dissociation from T cells and T cell precursors. J Immunol. 1983 Jun;130(6):2843–2848. [PubMed] [Google Scholar]



